The Role of Epicardial Adipose Tissue in Atrial Fibrillation: A Narrative Review
Authors: Joseph V. Pergolizzi, Jr., Jo Ann LeQuang*,
NEMA Research, Inc., Naples, Florida, United States
*Corresponding author: Jo Ann LeQuang, NEMA Research, Inc., Naples, Florida, United States; E-mail: joannlequang@gmail.com
Received: 16 February 2023; Accepted: 23 February 2023; Published: 27 February 2023
Citation: Pergolizzi Jr. JV, LeQuang JA (2023) The Role of Epicardial Adipose Tissue in Atrial Fibrillation: A Narrative Review 21st Century Cardiol, Volume 3 (1): 130
Abstract
Fatty tissue deposits around the epicardium have been tentatively associated with atrial fibrillation (AF), but the mechanisms remain unknown. Animal studies are not particularly helpful because most rodents have comparative little epicardial adipose tissue (EAT) while humans have a great deal. The metabolic activity of adipose tissue may play a role, because EAT produces pro-inflammatory and pro-fibrotic mediators. EAT infiltration of the myocardium is fairly common even in healthy people, but EAT infiltration of the left atrial appendage has a positive correlation to the severity of myocardial fibrosis. The volume of EAT in the heart may also be predictive and may emerge as a possible biomarker for early-onset arrythmias. EAT has the ability to change the electrophysiologic properties of myocytes and its neural network is only beginning to be elucidated. Myocytes function in an extracellular matrix maintained by fibroblasts, and their intercommunication appears to likewise influence electrophysiologic activity of the heart. AF patients are known to have more extracellular vesicles than those without AF and these vesicles appear to facilitate intercellular communications that regulate various physiologic processes, including supporting coagulation, which may influence stroke risk. The volume of EAT in the left atrium may be a marker for AF recurrence in patients undergoing catheter ablation, but the role of EAT in postoperative AF is not clear. This was a narrative review on our current limited but expanding understanding of EAT in the human heart and its potential links to AF.
Keywords:
Adipokines; Atrial fibrillation; Brown fat; Epicardial adipose tissue; White fat
Introduction
It is known that fatty deposits around the epicardium pose a risk for the development and progression of atrial fibrillation (AF), but less is known about why or how [1,2]. While visceral adipose tissue has long been associated with cardiovascular disorders, the specific role of epicardial adipose tissue (EAT) and other forms of adipose tissue around the heart is less well studied [3]. Animal models studying EAT have limited applicability since the amount and function of EAT varies widely among species, such that rodents have very little EAT and humans have a great deal [4]. The underlying mechanisms by which AF arises and progresses are not fully elucidated but there are clear connections between AF and EAT. The aim of this study was to explore recent findings in the literature relating on this topic of much current interest.
AF involves disorganized ectopic electrical activity originating in the atria and conducted by an atrial substrate, which may arise due to localized tissue damage [5]. Prolonged arrhythmic activity may cause atrial remodeling that can then support further fibrillation, leading to the old clinical adage describing this vicious cycle, ?AF begets AF? [6]. Although AF is sometimes described as a progression or spectrum, not all patients pass through these phases in order or even experience all of them. Paroxysmal AF may self-terminate while persistent AF is more long-lasting and may require medical intervention in the form of electrical or pharmacological treatment to convert back to sinus rhythm. Some AF is medically refractory and may be described as permanent or chronic. AF may exist in the setting of valvular disease or apart from it. Furthermore, AF may arise spontaneously after cardiac or other surgeries [7]. Risk factors for progression of AF have been well described in the literature: older age, heart failure, hypertension, chronic kidney or pulmonary diseases, type 2 diabetes mellitus (T2DM), a history of stroke, and an oversized left atrium.8 Therapeutic interventions have been primarily based on strategies of rate versus rhythm control [8]. AF may cause symptoms, even debilitating ones, but many patients with AF remain asymptomatic [9].
A particular risk with AF is that a thrombus can form, typically in the left atrial appendage, giving rising to stroke via peripheral embolization [10]. In fact, AF is a source of considerable morbidity and mortality by increasing a patient?s risk of stroke by five-fold [11].
Methods
This narrative review is based on a search of the peer-reviewed literature. The authors searched ?epicardial adipose tissue and atrial fibrillation? with no delimiters in PubMed and found 300 articles. We excluded articles not in English, not relevant to EAT or AF, as well as posters, abstracts, correspondence, patents, or other non-peer-reviewed materials. We included articles that dealt with the dual topics of EAT and AF. We searched Google Scholar as well, where there was considerable overlap with our PubMed results. While we imposed no temporal delimiters on our search, it was observed that most materials were written on or after 2017.
The authors also searched ?left atrial appendage epicardial adipose tissue? and retrieved 21 results, of which two were clinical studies. For Table 2, a literature search was conducted seeking clinical trials, randomized clinical trials, or meta-analyses using the keywords ?atrial fibrillation and epicardial adipose tissue.? This duplicated some results from the above search but it provided us with 15 specific trial results, of which 10 were used in the table. The three excluded studies were not relevant to our topic. The bibliographies of key articles were also searched as was general background information and statistics on AF.
Results
Adipose tissue depots in and around various areas of the heart have been described in the literature (see Table 1). Epicardial adipose tissue (EAT) is increasingly being recognized by its topography as unique and distinct from other fatty tissue in the body. By virtue of its location, it provides a degree of cushion for the heart muscle and this fatty layer may protect the coronary vessels from torsion during the heart?s depolarization and repolarizations [3]. The chemical composition of EAT and its lipogenicity may provide energy to the heart and offer protection against potentially lipotoxic exposure to free fatty acids [4]. EAT is involved in thermogenesis and confers cryoprotection to the heart [4]. The immunological and other cells produced by EAT include pro-inflammatory mediators, pro-fibrotic mediators, and other neurotransmitters, some of which may play important roles in arrhythmogenesis [4].
| Name | Description |
|---|---|
| Epicardial adipose tissue (EAT) | Derived from mesenchymal epicardial cells, it is located between the visceral layer of the pericardium (within the pericardial sac) and the myocardium with no intervening fibrous or other layer. It accounts for about 20% of heart weight. |
| Intramyocardial adipose tissue | Adipocytes residing in the myocardium |
| Intrathoracic adipose tissue | Visceral adipose tissue of the chest, including pericardial and epicardial adipose tissue plus other fatty deposits inside the chest but excluding subcutaneous fat |
| Paracardial adipose tissue | Located on the exterior of the fibrous portion of the pericardium, but the term is falling into disuse because of lack of clarity |
| Pericardial adipose tissue | Epicardial plus paracardial adipose tissue, that is the fatty tissue of the thorax that surrounds the heart and pericardial sac |
| Perivascular adipose tissue | Adipose tissue located outside of the blood vessels and present to some extent around all arteries except the cerebral artery and microcirculatory vessels |
Table 1: Types of cardiac adipose tissue [4,12]. Note that some of these terms are becoming more historical than contemporary, for example, ?paracardial adipose tissue.?
EAT is located between the visceral layer of the serous pericardium and the surface of the heart, putting it in direct contact with the myocardium. It possesses properties distinct from those of pericardial adipose tissue, which is found inside the visceral and parietal layers of the serious pericardium, in other words, not in direct contact with the myocardium [13]. While the volume of EAT is relatively small in certain animals, such as mice, in healthy humans, EAT can cover 56% to 100% of the cardiac circumference and comprise up to 20% of the weight of the heart [14]. Women have significantly lower volumes of total EAT compared to men (p<0.001), but the ratio of peri-atrial adipose tissue to EAT was significantly higher in women (p=0.009) than men [15]. See Figure 1.
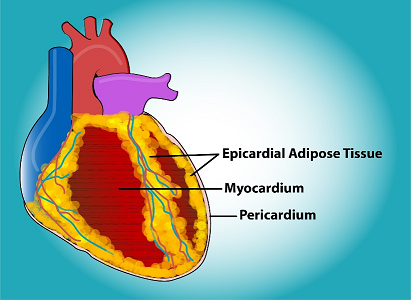
Figure 1: Epicardial adipose tissue is located within the pericardial sac and adjacent to the myocardium. Original art by Todd Cooper of The Coyote Studios.
While EAT infiltration into the myocardium is relatively prevalent, even in healthy individuals, [16] EAT infiltration of the left atrial appendage has been positively correlated with myocardial fibrosis severity [16]. The location of EAT with respect to the left atrium can indicate the existence and severity of a so-called low-voltage zone, defined an area during sinus rhythm with ?0.5 millivolt on a bipolar electrogram [17] The volume of EAT may also predictive, because it is larger in those with paroxysmal AF than in those with normal sinus rhythm, making it a potential marker for early-onset arrhythmia [18].
EAT derives from the mesenchymal transformation of epicardial cells, which become vascularized by branches extended from the coronary arteries [14]. EAT is thought to act as a localized modulator of cardiovascular disease by secreting various factors in paracrine fashion that affect cardiomyocytes and the vasculature [12]. All adipose tissue is biologically active, and EAT is known to be involved in lipid and energy homeostasis [1]. Its ability to metabolize and secrete free fatty acids may explain its cardioprotective properties. Adipose tissue also produces bioactive molecules, including pro-inflammatory mediators and adipocytokines. EAT from patients with ischemic forms of heart disease tends to be higher in pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-?), which in theory might promote insulin resistance [1]. In contrast to subcutaneous adipose tissue, EAT expresses more of the uncoupling protein-1 (UCP-1), located in the mitochondrial inner membrane [1]. UCP-1 has been described as the phenotypic signature of brown ?healthy? adipose tissue, which serves to generate heat [1]. It has been speculated that EAT may be thermally protective of the myocardium since UCP-1 is a specific biomarker for nonshivering thermogenesis in brown adipocytes [19]. From fatty tissue samples taken from 50 patients undergoing open-heart surgery (44 coronary artery bypass grafts, 6 heart valve replacements), UCP-1 expression was five times greater in epicardial than substernal fat; UCP-1 expression was minimal in subcutaneous fat deposits [19]. The UCP-1 expression in EAT increases with body mass index, decreases with age, has no relationship to epicardial fat volume or waist circumference, and is similar in men and women and in patients on or not on statin therapy [19]. This suggests that EAT functions more like brown fat as a defender of myocardial tissue and coronary vessels, with this benefit lessening with advancing age [19,20].
Pericardial fat tends to be described as brown or beige with health, whitening with age and/or disease. Brown and beige fat use glucose and lipids to produce heat and offer cardioprotective benefits [12]. Brown fat transitions to less-healthy white fat in obesity, and this brown-to-white transition appears to influence the development of cardiovascular disease [12]. This pathological change in the adipose tissue may contribute directly to metabolic disorders and atherosclerosis. Unlike brown fat, white adipose tissue lacks UCP-1 [12]. The potential for ?browning? white adipose tissue around the heart is currently a field of investigation [12].
Infants have a higher proportion of brown fat which decreases as they mature [21]. Over time, brown fat transitions to white fat in a gradual process. Recently, some fat has been described as beige or brown-in-white (?brite?), characterized by white adipocytes that express brown genes, including thermogenetic properties [20,22]. Beige or brite fat may change to white fat with removal of a thermal challenge or other factors [23]. Because EAT expresses UCP-1, it is typically considered a beige or brite fat, [23] but with obesity, EAT may transition to a white fat and no longer express UCP-1 [12]. Conversely, white fat may also revert back to beige or brown fat. The so-called ?rebrowning effect? can occur with EAT when genes associated with brown adipocytes are upregulated accompanied by decreased inflammation [24].
Brown fat tends to have more gap junctions compared to white fat, [25] but it remains unknown whether adipocytes can forge functional gap junctions with nearby fibroblasts or myocytes. If such coupling of dissimilar cells is possible, it suggests that the electrophysiologic properties of myocytes may be subject to modulation [16]. Should such heterocellular gap junctions form, they might contribute to heterogeneous electrophysiologic function in atrial tissue, posing a potential risk for AF [16]. This finding would make the ratio of relative volumes of adipocytes to myocytes important, because a large number of adipocytes coupled to a myocyte could impede myocyte depolarization and increase the threshold of the action potential [16]. Adipocytes are much larger in volume than myocytes and both are larger than fibroblasts so a high volume of adipocytes is possible. The presence of fat within an adipocyte may further impede electrical conduction speed [16].
Adipokines are a type of signaling molecule produced by adipose tissue whose role is to modulate the physiology of myocytes and fibroblasts. In a healthy heart, adipokines serve to protect the heart from inflammation and fibrosis [16]. However, adipokines can abruptly reverse roles when reactive oxygen species (ROS) is produced, taking on a pro-inflammatory and profibrotic role which can damage epicardial and myocardial tissue [16]. Furthermore, EAT has the ability to alter electrophysiologic properties of myocytes [16] and the interplay between myocytes and nonmyocytes in this connection may play a crucial role in AF. The adipokines in EAT appear to modulate leukocytes and fibroblasts in the fatty tissue and leukocytes, fibroblasts, and myocytes in the myocardium. Among these many adipokines are omentin, apelin, adiponectin, resistin, adipose fatty-acid-binding protein, vistafin, leptin, and others [16]. The neural network within EAT releases other neurotransmitters, such as acetylcholine and vasoactive intestinal protein [26]. A proposed theory investigated in an animal model is that the neurotransmitters in EAT regulate the excitability of the myocytes, which could trigger AF [27]. Cholinergic nerves of the EAT have been implicated in postoperative AF [28]. This was shown when an injection of botulinum toxin into EAT in cardiac surgery patients blocked acetylcholine release from the parasympathetic nervous system and reduced the incidence of postoperative AF in the first 30 days after surgery [29]. This injection conferred protection against AF for a year after surgery and may be due to modification of the EAT secretome.
Epicardial Myocytes
In the healthy heart, the myocardial tissue preserves a highly organized sequence of action potentials across the tissue to promote coherent cardiac depolarizations [13]. Healthy myocytes safeguard this property, but may be disrupted by inflammatory processes, coronary artery disease, ischemia, and other conditions [13]. Myocytes function in an extracellular matrix like a scaffold; this matrix is maintained by fibroblasts, which can adapt the scaffold as needed [30]. Myocytes and fibroblasts communicate with each other and it appears that they can also influence the electrophysiologic properties of the heart muscle [31]. Myocytes are disproportionately distributed in the heart. The human heart has far more fibroblasts than myocytes, but the myocytes are considerably larger and thus compose a greater volume. This volume difference is much more marked in the ventricles than the atria [13,16].
Large Extracellular Vesicles
Naturally released by diverse types of cells including the EAT, extracellular vesicles (EV) lack a nucleus and therefore cannot replicate. In the past, these particles have been described under a variety of different names that have fallen into disuse: exosomes, ectosomes, and microparticles [32]. The main function of EVs is to facilitate cell-to-cell communication pathways [33]. They have been implicated in the development and prolongation of AF and may be considered as potential biomarkers [34-36].
In a study of cultured EAT specimens taken from 62 patients (32 of whom had AF) during cardiac procedures, inflammation, fibrosis, and apoptosis were observed in all of the EAT cultures [37]. However, AF patients exhibited both a greater number of EVs and these EVs possessed larger amounts of pro-inflammatory cytokines and pro-fibrotic microRNA than those from patients without AF. Furthermore, the cultures from AF patients promoted a sustained form of re-entry in cardiomyocytes derived from pluripotent stem cells [37].
It was speculated that large EVs may possess a pro-coagulant function that would limit or suppress stroke [10]. In a study of 836 AF patients (280 had a history of stroke) from the ARISTOTLE trial, EVs were measured using flow cytometry and compared to a cohort of unselected individuals aged 70 years (n=1,007).38 EVs were taken from platelets, leukocytes, erythrocytes, and inflammatory endothelial cells. Patients with AF had significantly higher levels of EVs compared to the control group, but none of the EVs measured at baseline could be associated with a later stroke in AF patients. For that reason, the study concluded that EVs probably were not mediators of stroke in AF patients [38]. However, the problem may be more complex than it initially appears. In a study of 58 patients, of whom 49% had permanent AF, 34% non-permanent AF, and 17% no history of AF, blood was sampled from three specific cardiac locations: in the right atrium, in the left atrium, and in the left atrial appendage [10]. Using flow cytometry, it was found that large EVs were significantly more common in the left atrial appendage (LAA) of permanent AF patients compared to those with non-permanent AF. Platelet-derived large EVs were also significantly more numerous in the LAA of patients with any type of AF. Thus, platelet-derived large EVs were more plentiful in the LAA of permanent AF patients, suggesting that these large EVs might be involved in thrombus formation within the LAA [10]. The anatomical and electrophysiologic properties of the LAA are associated with its capacity to form thrombi; in fact, 90% of thrombus formation in nonvalvular AF patients occurs in the LAA [39]. Even in patients with valvular AF, 57% of thrombus formation occurs in the LAA [40].
Since EVs facilitate intercellular communications associated with a variety of physiologic processes and are known to have pro-coagulation effects in the heart, their potential role in stroke is of great interest [41]. In a study of 25 patients, blood taken during open-heart surgery found that patients with a history of AF had higher EV levels than those without AF, [42] and a study by Zietzer A, et al. (2022) found that the type of AF, defined as permanent versus not-permanent, influences the distribution of EV sub-types in the LAA [10].
The Left Atrial Appendage
Due to its mechanical, anatomic, and electrophysiologic properties, the LAA is considered to the point of origin of most thrombi [39]. LAA morphology is highly variable among patients (see Figure 2) and AF can result in LAA remodeling, as was shown in a controlled retrospective study of 225 patients, of whom 76 had persistent and 70 had paroxysmal AF. Using medical records of echocardiograms and computed tomography, LAA morphology was classified as ?chicken wing? or ?non-chicken-wing? [43]. Persistent AF patients were more likely to have ?non-chicken-wing? LAA morphology [43]. However, in a study of 84 patients who underwent radiofrequency ablation for AF, those with ?chicken-wing? LAA morphology had a significantly higher incidence of AF recurrence (68.2%, p<0.01) [44]. In general, it appears that ?chicken wing morphology? of the LAA conferred a degree of relative protection against thrombus formation [45].
Figure 2: There is considerable variation in left atrial appendage morphology and patients with persistent AF. The four main shapes are described below. About 12% of patients have the chicken wing shape. Original art by Todd Cooper, The Coyote Studios.
Pro-inflammatory and profibrotic cytokines and adipokines in EAT may be located adjacent to the pulmonary veins and close to the LAA. In a study of 61 consecutive mitral valve surgery patients, of whom 15 had AF, the AF group had significantly higher rates of tyrosine hydroxylase and choline acetyltransferase in the LAA, in the EAT, and in the pulmonary vein muscle sleeve myocardium. These pro-inflammatory and profibrotic mediators may be associated with LAA remodeling and arrhythmogenesis [46]. The relationship between the LAA and EAT remains to be elucidated. A thickened layer of EAT was associated in one study (n=202) with low LAA emptying velocity, which might increase the risk of thromboembolism [47]. The morphology of the LAA along with its trabeculae likely play a role in promoting or discouraging thrombus formation [48].
The Association of EAT and AF
Despite evidence that the volume of EAT can be correlated to AF, it is not clear if there is a dose-response relationship, that is, if larger volumes confer a proportionately higher risk. In a study of 326 patients in Japan (n=326, of whom 214 had AF) the volume of EAT had a strong association to the incidences of both paroxysmal and persistent forms of AF, and, in this connection, the volume of the left atrium played a role in the incidence of persistent, but not paroxysmal, AF [49]. This study excluded patients with coronary artery disease, so these results may not be generalizable to a real-world clinical population. In that study, the cut-off value for the volume of EAT was 64 mL/m2 for persistent AF but 55 mL/m2 for paroxysmal AF [49]. These cut-off values could be used independently from other AF risk factors to predict the risk of persistent or paroxysmal AF [49].
The volume of EAT on the left atrium appears to be a risk marker for AF recurrence following catheter ablation [50]. In patients undergoing pulmonary vein isolation procedures, a higher EAT volume on the left atrium was associated with conduction disorders [51]. In a study of 105 paroxysmal AF patients who underwent electro-anatomical mapping, P-wave duration was longer and left-atrial conduction velocity slower in patients with greater EAT volume (p<0.001, both). In fact, p-wave duration and conduction velocity could be correlated with left atrial EAT (?=0.367, p<0.001 and ?=-0.566, p<0.001, respectively) [51].
The role of EAT in postoperative AF remains unclear. In a study of 83 patients undergoing coronary artery bypass graft surgery and/or a form of valvular surgery, 43 patients developed postoperative AF after the procedure. Using multivariable analysis, it was found that the volume of the LA itself, rather than the volume of EAT, was an independent predictor of postoperative AF [52]. However, the ratio of left-atrial EAT to total EAT could be associated with postoperative AF in a study of cardiac surgery patients (n=77) [53]. A variety of clinical studies have assessed the relationship between EAT volume and AF. See Table 2.
Table 2: Clinical studies, randomized clinical trials, and meta-analyses exploring the relationship of EAT to AF incidence. Studies appear in alphabetical order by first author in each category.
|
Study |
Endpoint |
Subjects |
Key Findings |
|
EAT Volume and AF |
|||
|
Gaeta M (2017) [54] Meta-analysis |
AF |
7 studies |
The volume of EAT is significantly greater in all AF than non-AF patients, but persistent AF patients had significantly greater volumes of EAT than paroxysmal AF patients |
|
Wong CX (2016) [55] Meta-analysis |
AF |
63 observational studies (n=352,275) |
The association between EAT and AF was greater than the association of abdominal or overall adiposity with AF |
|
Zhu W (2016) [56] Meta-analysis |
AF |
10 case control studies |
The volume of EAT in total and around the left atrium were significantly associated with AF |
|
EAT and Post-Procedural AF Recurrence |
|||
|
Canpolat U (2016) [57] RCT |
AF |
234 AF patients undergoing balloon cryoablation |
Greater EAT thickness was an independent predictor of AF recurrence following balloon cryoablation |
|
Chen J (2022) [58] Meta-analysis |
AF |
10 studies (n=1,840) of AF patients undergoing catheter ablation |
Greater EAT thickness was a strong predictor of AF recurrence following catheter ablation and may be useful as a marker prior to ablation to determine who will benefit from procedure |
|
Nagashima K (2011) [59] CT |
AF |
40 AF patients undergoing catheter ablation |
EAT volume is larger in AF than non-AF patients and may be useful as a predictor of AF recurrence following AF catheter ablation |
|
EAT and Postoperative AF |
|||
|
Pokushalov E (2015) [28] RCT |
AF one year after surgery |
60 AF patients undergoing CABG |
Botulinum toxin injection into EAT during CABG reduced AF after surgery and for one year postoperatively with no serious side effects |
|
Pokushalov E (2014) [60] RCT |
POAF |
60 AF patients undergoing CABG |
Botulinum toxin injection into EAT during CABG reduced POAF (7% in botulinum groups versus 30% of controls) |
|
Sha R (2021) [61] Meta-analysis |
POAF |
10 studies |
Both EAT volume and thickness was greater in patients who experienced POAF following cardiac surgery. |
|
White CM (2007) [62] RCT (AFIST-III) |
POAF |
180 CABG patients, of whom 5% had history of AF |
Maintaining the anterior fat pad did not reduce incidence of POAF |
|
AF: atrial fibrillation; CABG: coronary artery bypass graft (procedure); CAD: coronary artery disease; EAT: epicardial adipose tissue; POAF: postoperative atrial fibrillation; RCT: randomized controlled trial; T2DM: type 2 diabetes mellitus |
|||
|
Study |
Endpoint |
Subjects |
Key Findings |
|
EAT Volume and AF |
|||
|
Gaeta M (2017) [54] Meta-analysis |
AF |
7 studies |
The volume of EAT is significantly greater in all AF than non-AF patients, but persistent AF patients had significantly greater volumes of EAT than paroxysmal AF patients |
|
Wong CX (2016) [55] Meta-analysis |
AF |
63 observational studies (n=352,275) |
The association between EAT and AF was greater than the association of abdominal or overall adiposity with AF |
|
Zhu W (2016) [56] Meta-analysis |
AF |
10 case control studies |
The volume of EAT in total and around the left atrium were significantly associated with AF |
|
EAT and Post-Procedural AF Recurrence |
|||
|
Canpolat U (2016) [57] RCT |
AF |
234 AF patients undergoing balloon cryoablation |
Greater EAT thickness was an independent predictor of AF recurrence following balloon cryoablation |
|
Chen J (2022) [58] Meta-analysis |
AF |
10 studies (n=1,840) of AF patients undergoing catheter ablation |
Greater EAT thickness was a strong predictor of AF recurrence following catheter ablation and may be useful as a marker prior to ablation to determine who will benefit from procedure |
|
Nagashima K (2011) [59] CT |
AF |
40 AF patients undergoing catheter ablation |
EAT volume is larger in AF than non-AF patients and may be useful as a predictor of AF recurrence following AF catheter ablation |
|
EAT and Postoperative AF |
|||
|
Pokushalov E (2015) [28] RCT |
AF one year after surgery |
60 AF patients undergoing CABG |
Botulinum toxin injection into EAT during CABG reduced AF after surgery and for one year postoperatively with no serious side effects |
|
Pokushalov E (2014) [60] RCT |
POAF |
60 AF patients undergoing CABG |
Botulinum toxin injection into EAT during CABG reduced POAF (7% in botulinum groups versus 30% of controls) |
|
Sha R (2021) [61] Meta-analysis |
POAF |
10 studies |
Both EAT volume and thickness was greater in patients who experienced POAF following cardiac surgery. |
|
White CM (2007) [62] RCT (AFIST-III) |
POAF |
180 CABG patients, of whom 5% had history of AF |
Maintaining the anterior fat pad did not reduce incidence of POAF |
|
AF: atrial fibrillation; CABG: coronary artery bypass graft (procedure); CAD: coronary artery disease; EAT: epicardial adipose tissue; POAF: postoperative atrial fibrillation; RCT: randomized controlled trial; T2DM: type 2 diabetes mellitus |
|||
Using multi-slice spiral computed tomography (CT) to assess the total volume of EAT and the left-atrial EAT, 207 patients (125 with AF, 82 in sinus rhythm) were evaluated. Among the subjects with AF, 80 had paroxysmal and 45 persistent AF [63]. In this study from China it was found that EAT, whether in totality or specific to the left atrium, had a significantly larger volume in any AF group compared to the sinus rhythm patients (p<0.01, all). Using logistic regression analysis, it can be shown that the total volume of EAT or the volume of EAT in the left atrium were independent related factors of AF and according to the Pearson correlation analysis, total EAT volume and left-atrial EAT volume could be positively correlated to the left atrial diameter (r=0.466 and r=0.290, respectively, p<0.01 both) [63]. See Table 3.
Table 3: Based on data obtain from Zhu and colleagues, the volume of EAT in total and specific to the left atrium were recorded in patients with normal sinus rhythm and AF. All volumes are cubic centimetres. Data are based on 207 patients of whom 72 had normal sinus rhythm (first column); 125 had any type of AF (second column). Of this AF population the fourth and fifth columns differentiate between paroxysmal and persistent AF [63].
|
|
Sinus rhythm n=82 |
AF overall n=125 |
Paroxysmal AF n=80 |
Persistent AF n=45 |
|
All data expressed in mL/m2 |
||||
|
Volume of EAT (total) |
92.2±32.1 |
136.0±46.0 |
134.2±46.3 |
140.1 ± 52.6 |
|
Volume of EAT in the left atrium |
27.1±7.5 |
39.2±19.2 |
35.9±17.0 |
45.1±21.5 |
EAT and Cardiac Electrophysiology
The atrial substrate necessary for AF may be the byproduct of cytokine activity in the EAT secretome. Implicated among these cytokines is a member of the super-family of transforming growth factor ? (TGF-?) known as activin A [1]. Activin A has known pro-fibrotic effects [64]. In a study of 89 cardiac surgery patients, the expression of activin A by the EAT served as an independent predictor of those who would develop postoperative AF, particularly, but not exclusively, for those with valvular heart disease [65]. These same conclusions were reached in a study of 124 on-pump coronary artery bypass graft patients, where thicker EAT predicted elevated risk for postoperative AF [66]. Thus, the EAT secretome may promote atrial fibrosis via the expression of activin A [67].
Matrix metalloproteinases (MMPs) help to modulate cytokines throughout the body and may induce cardiac remodeling as well as contribute to certain disease processes, such as arthritis and cancer. MMPs are involved in regulating basement member components, including collagen fibers. It has been shown in a murine model that MMP-2 and MMP-7 are upregulated during AF and may be associated with the accumulation of interstitial fibrosis [68]. MMP-8 is expressed in EAT and has been associated with atherosclerotic plaque formation [69].
The pathogenesis of AF involves an inflammatory response, and C-reactive protein levels are double in those with AF than those without and higher in those with chronic AF versus paroxysmal AF [1]. Pro-inflammatory cytokines such as interleukin-6 (IL-6), IL-8, and TNF-? are produced by EAT and have also been associated with AF [1].
The pro-fibrotic activity of EAT can combine with these inflammatory responses to create a vulnerable substrate. The transition from fatty infiltrates extending into the myocardium to fibrofatty infiltrates and a thickening of the surface of the adipose layer are associated with fibrosis and have been linked to the development of AF [70]. Macrophagy promotes fibrosis in adipose tissue because the secretion of TGF-? stimulates a differentiation of pre-adipocytes that, in turn, stimulates myofibroblast production [71]. In a study of human hearts, more fibrotic growth occurred in areas where the myocardium was in direct contact with adipose tissue than elsewhere [72]. This is particularly true in the case of cardiac trauma, during which adipocytes and fibroblasts of the myocardium become activated, migrate toward the myocardium, and differentiate into fibroblasts or adipocytes [73]. Additionally, fibrofatty infiltrates in EAT are associated with a slower conduction time for the action potential and conduction heterogeneity across the atrial myocardium, [74] both of which could contribute to AF or its progression.
The electrophysiologic property of re-entry is the underlying mechanism of many tachyarrhythmias, but the role and nature of re-entrant circuits in AF remain unclear. Re-entry rotors, independent wavelets, and a newer double-layer hypothesis of dissociation have been offered to better explain the unique mechanisms underlying AF [75-78]. Longitudinal dissociation describes the asynchronous propagation of waveforms along the longitudinal axis of the atrioventricular conduction pathway, permitting various forms of re-entry to arise. Longitudinal dissociation in atrial tissue in older individuals suggests a progressive, age-related electrical dissociation of the side-by-side atrial muscle bundles. A recent qualitative analysis of intra-atrial conduction disturbances during AF reported that the primary feature of the substrate associated with long-standing AF was longitudinal dissociation, presenting as lines of conduction block running parallel to the atrial muscles. On the other hand, rotors or multiple foci that could explain long-standing AF have not yet been discovered [78]. Focal fibrillatory waves sometimes associated with AF may actually be waves originating at deeper layers of the musculature breaking through the epicardium [79]. AF is increasingly being described as a three-dimensional condition [80].
The healthy atrial myocardium depolarizes in a unified and coherent pattern as the waveform propagates across the tissue; however, when this depolarization becomes disorganized or patchy, it may allow for microcircuits to form [1]. EAT forms around the epicardium but may over time penetrate deeply into the epicardial layer and from there into the myocardial tissue itself, promoting atrial disorganization and substrate formation [81].
The atrial substrate typically exhibits myofibroblasts along with de-differentiated and dystrophic myocytes, but it is not known how these cells remodel [82]. Adipose tissue contains stem cells in the stroma fraction, and these cells are able to differentiate into adipocytes, myofibroblasts, or cardiomyocytes [83]. In other words, EAT itself may be the tissue providing the precursor cells used in structural atrial remodeling [1].
Obesity and AF
The association between obesity and AF was established in the Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up (LEGACY) study which evaluated 1,415 consecutive AF patients [84]. The majority of patients (58%) had a body mass index ? 27 kg/m2 and were offered an intervention consisting of diet and risk factor management. Among those patients who lost 10% or more of their body weight, 88% reversed from persistent AF to paroxysmal or no AF (p<0.001). Overall, the more weight lost, the greater the degree of freedom from AF [84]. This study evaluated total body weight, not EAT.
The volume of the EAT depot is considered an index of cardiac obesity, [4] and might be a more accurate predictor of AF than body mass index [85]. In a study of 3,217 participants from the Framingham Heart Study Offspring and Third-Generation Cohorts, subjects underwent a multidetector CT procedure to measure pericardial, intra-thoracic, and visceral adipose tissue volumes and its relationship to AF, adjusting for established AF risk factors. Note that pericardial fat included both paracardial adipose tissue and EAT. Of the total cohort, 54 had been diagnosed with AF. In statistical models, pericardial fat but not intra-thoracic or visceral abdominal fat was associated with AF [86]. However, a subsequent analysis of 2,135 participants from the same trial failed to find significant associations between ectopic cardiac adipose tissue deposits and incident AF [87].
Future Directions
The genetic component of EAT in AF is of great interest. CircRNA, a type of noncoding RNA, has been detected in EAT and may be involved in the pathogenesis of disorders that predispose patients to heart failure [88] and AF [89]. By definition, a non-coding RNA (ncRNA) does not translate into a protein. These ncRNA are ?master regulators? that govern the genome at the highest level and manage numerous cellular processes. The dysregulation of ncRNAs have been associated with such adverse consequences as cancer. One type of ncRNA is of particular interest: circular RNA (circRNA), which forms a closed continuous loop rather than a linear strand. Exosomes bearing circRNA have been identified as having been produced by EAT [89]. Analyzing EAT from 12 patients (50% had AF), RNA sequencing was able to identify 2,159 circRNAs, of which 528 were upregulated while 579 were downregulated [89]. This number included 10 circRNA forms that existed only in the setting of AF, all of which were upregulated [89]. These circRNAs modulate microRNA in heart disease and the interaction among circRNA, microRNA, and messenger RNAS (mRNA) may play a role in AF [90]. Plasma circRNA has been studied for its potential role as a biomarker for AF prognosis [91].
Another mediator produced by the EAT of growing interest to the study of AF is YKL-40, which takes its name from its molecular weight and its three terminal amino acids: tyrosine (Y), lysine (K), and leucine (L). Expressed by the chitinase-3-like protein 1 (CHI3L1) gene, YKL-40 is emerging as an important new biomarker for heart disease in general [92]. YKL-40 indicates the presence of inflammation, remodeling, and fibrosis and it is found at elevated levels in people with AF [92]. YKL-40 is highly expressed in the EAT of AF patients, in particular those with higher body mass index [92].
Discussion
Our understanding of the unique characteristics of EAT and its associations to both the pathogenesis and progression of AF has created promising new areas of research for AF. AF is the most common arrhythmia, yet its underlying mechanisms remain to be elucidated. AF is complex, as it exists in a multiplicity of forms, such as postoperative AF, paroxysmal AF, persistent AF, valvular AF, nonvalvular AF, chronic AF, and even intractable forms, which may or may not be related to each other. Safe and effective AF treatments in the form of ablative procedures, pharmacological treatments, electro-cardioversion and other forms of device-based therapy, as well as lifestyle modifications, have all been explored, but not all patients respond to these therapies and even after initial success, AF recurrence is not uncommon. Perhaps the best illustration of how challenging AF is to treat is the ongoing clinical debate about whether to address rate control or rhythm control in AF management. Yet clinicians are undivided in the conviction that AF should be treated, if at all possible, as it is closely associated with morbidity and mortality.
The differentiation of EAT as distinct among other forms of fat in or around the heart represents an important scientific milestone. EAT may well provide us with effective biomarkers for AF vulnerability, risk, and prognosis. The volume of EAT alone may have an important predictive value and could serve as a drug target far beyond that of management of AF. A study of dapagliflozin demonstrated that the volume of EAT may be decreased pharmacologically [93]. A study of 2,482 subjects found EAT volume could be correlated to a number of conditions, including coronary artery disease, fatty liver disease, cardiac abnormalities, metabolic syndrome, and insulin resistance [94]. EAT thickness has been associated with the QT interval, with thicker layers indicative of more prolonged QT intervals in patients with arterial hypertension [95].
This narrative review has several limitations. The subject of AF is broad and we do not make rigorous distinctions among paroxysmal AF, persistent AF, postoperative AF, and other types of the arrhythmia, which may vary in terms of how EAT affects them. Much of our understanding of EAT is relatively new and therefore subject to change as research progresses. This is an important albeit fragmented area for research, few studies have been conducted, and conventional animal models are not particularly serviceable for research. Nevertheless, EAT and its association with AF opens up a promising new area of investigation in our ongoing efforts to better understand and manage AF.
Conclusion
While the role of EAT in the pathogenesis of AF remains to be elucidated, it is clear that EAT is a special type of cardiac fat depot which chemically interacts with the myocytes in ways that may encourage development of an atrial substrate and alter electrophysiologic properties. While obesity is considered a risk factor for AF, it is cardiac obesity and EAT volume in particular that may be a more specific predictor and biomarker. Further study of EAT and its biochemical interactions with the heart muscle are warranted and may help to elucidate the underlying mechanisms of AF.
References
1. Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovascular research. 2014 May 1;102(2):205-13. https://doi.org/10.1093/cvr/cvu045
2. Xu X, Zhou Q, Ren C, Wang F, Chen Y, Sun H. Evaluation of Patients with Angiographically-Confirmed Coronary Artery Disease to Investigate the Association Between Epicardial Fat Thickness and Atrial Fibrillation. Medical science monitor: international medical journal of experimental and clinical research. 2022;28:e936446-1. https://doi.org/10.12659/MSM.936446
3. Antonopoulos AS, Antoniades C. The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles. The Journal of physiology. 2017 Jun 15;595(12):3907-17. https://doi.org/10.1113/JP273049
4. Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. American journal of physiology-endocrinology and metabolism. 2012 Oct 15;303(8):E937-49. https://doi.org/10.1152/ajpendo.00061.2012
5. Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circulation research. 2014 Apr 25;114(9):1483-99. https://doi.org/10.1161/CIRCRESAHA.114.302226
6. Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. European journal of internal medicine. 2009 Nov 1;20(7):672-81. https://doi.org/10.1016/j.ejim.2009.07.011
7. Aguilar M, Nattel S. Postoperative atrial fibrillation after noncardiac surgery: maybe not so benign after all. Canadian Journal of Cardiology. 2019 Nov 1;35(11):1423-5. https://doi.org/10.1016/j.cjca.2019.08.013
8. Deng H, Bai Y, Shantsila A, Fauchier L, Potpara TS, Lip GY. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: a systematic review. Clinical Research in Cardiology. 2017 Oct;106:813-23. https://doi.org/10.1007/s00392-017-1123-0
9. Dilaveris PE, Kennedy HL. Silent atrial fibrillation: epidemiology, diagnosis, and clinical impact. Clinical cardiology. 2017 Jun;40(6):413-8. https://doi.org/10.1002/clc.22667
10. Zietzer A, Al-Kassou B, Jamme P, Rolfes V, Steffen E, Bulic M, Hosen MR, Goody PR, Tiyerili V, Zimmer S, Schrickel JW. Large extracellular vesicles in the left atrial appendage in patients with atrial fibrillation—the missing link?. Clinical Research in Cardiology. 2022 Jan;111(1):34-49. https://doi.org/10.1007/s00392-021-01873-4
11. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Medical Clinics of North America. 2008 Jan 1;92(1):17-40. https://doi.org/10.1016/j.mcna.2007.09.002
12. Aldiss P, Davies G, Woods R, Budge H, Sacks HS, Symonds ME. ‘Browning’the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. International journal of cardiology. 2017 Feb 1;228:265-74. https://doi.org/10.1016/j.ijcard.2016.11.074
13. Nalliah CJ, Bell JR, Raaijmakers AJ, Waddell HM, Wells SP, Bernasochi GB, Montgomery MK, Binny S, Watts T, Joshi SB, Lui E. Epicardial adipose tissue accumulation confers atrial conduction abnormality. Journal of the American College of Cardiology. 2020 Sep 8;76(10):1197-211. https://doi.org/10.1016/j.jacc.2020.07.017
14. Sacks HS, Fain JN. Human epicardial fat: what is new and what is missing?. Clinical and Experimental Pharmacology and Physiology. 2011 Dec;38(12):879-87. https://doi.org/10.1111/j.1440-1681.2011.05601.x
15. Zhu J, Zhuo K, Zhang B, Xie Z, Li W. Sex Differences in Epicardial Adipose Tissue: Association With Atrial Fibrillation Ablation Outcomes. Frontiers in Cardiovascular Medicine. 2022:1464. https://doi.org/10.3389/fcvm.2022.905351
16. Krishnan A, Chilton E, Raman J, Saxena P, McFarlane C, Trollope AF, Kinobe R, Chilton L. Are interactions between epicardial adipose tissue, cardiac fibroblasts and cardiac myocytes instrumental in atrial fibrosis and atrial fibrillation?. Cells. 2021 Sep 21;10(9):2501. https://doi.org/10.3390/cells10092501
17. Wang J, Sun X, Liu W, Zhu X, Zhu Y, Lin S, Chen H, Xu Y. Cardiac computed tomography-based epicardial adipose tissue assessment reveals association with electroanatomical voltage mapping in patients with atrial fibrillation. Heart, Lung and Circulation. 2022 Oct 1;31(10):1385-92. https://doi.org/10.1016/j.hlc.2022.07.001
18. van Rosendael AR, Smit JM, El’Mahdiui M, van Rosendael PJ, Leung M, Delgado V, Bax JJ. Association between left atrial epicardial fat, left atrial volume, and the severity of atrial fibrillation. Europace. 2022 Aug;24(8):1223-8. https://doi.org/10.1093/europace/euac031
19. Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, Karas J, Optican R, Bahouth SW, Garrett E, Wolf RY. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. The Journal of Clinical Endocrinology & Metabolism. 2009 Sep 1;94(9):3611-5. https://doi.org/10.1210/jc.2009-0571
20. Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H, Cinti S, Symonds ME. Adult epicardial fat exhibits beige features. The Journal of Clinical Endocrinology & Metabolism. 2013 Sep 1;98(9):E1448-55. https://doi.org/10.1210/endo.154.9.zee3482
21. Iacobellis G. Aging effects on epicardial adipose tissue. Frontiers in Aging. 2021 May 13;2:666260. https://doi.org/10.3389/fragi.2021.666260
22. Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. Journal of the American Heart Association. 2014 Mar 4;3(2):e000582. https://doi.org/10.1161/JAHA.113.000582
23. Altshuler-Keylin S, Shinoda K, Hasegawa Y, Ikeda K, Hong H, Kang Q, Yang Y, Perera RM, Debnath J, Kajimura S. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell metabolism. 2016 Sep 13;24(3):402-19. https://doi.org/10.1016/j.cmet.2016.08.002
24. Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. The Journal of clinical investigation. 2009 Nov 2;119(11):3329-39. https://doi.org/10.1172/JCI39228
25. Burke S, Nagajyothi F, Thi MM, Hanani M, Scherer PE, Tanowitz HB, Spray DC. Adipocytes in both brown and white adipose tissue of adult mice are functionally connected via gap junctions: implications for Chagas disease. Microbes and infection. 2014 Nov 1;16(11):893-901. https://doi.org/10.1016/j.micinf.2014.08.006
26. Lin YK, Chen YC, Chang SL, Lin YJ, Chen JH, Yeh YH, Chen SA, Chen YJ. Heart failure epicardial fat increases atrial arrhythmogenesis. International journal of cardiology. 2013 Sep 1;167(5):1979-83. https://doi.org/10.1016/j.ijcard.2012.05.009
27. Lin YK, Chen YC, Chen JH, Chen SA, Chen YJ. Adipocytes modulate the electrophysiology of atrial myocytes: implications in obesity-induced atrial fibrillation. Basic research in cardiology. 2012 Sep;107:1-1. https://doi.org/10.1007/s00395-012-0293-1
28. Pokushalov E, Kozlov B, Romanov A, Strelnikov A, Bayramova S, Sergeevichev D, Bogachev-Prokophiev A, Zheleznev S, Shipulin V, Lomivorotov VV, Karaskov A. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: one-year follow-up of a randomized pilot study. Circulation: Arrhythmia and Electrophysiology. 2015 Dec;8(6):1334-41. https://doi.org/10.1161/CIRCEP.115.003199
29. Couselo?Seijas M, López?Canoa JN, Agra?Bermejo RM, Díaz?Rodriguez E, Fernandez AL, Martinez?Cereijo JM, Durán?Muñoz D, Bravo SB, Velo A, González?Melchor L, Fernández?López XA. Cholinergic activity regulates the secretome of epicardial adipose tissue: Association with atrial fibrillation. Journal of Cellular Physiology. 2019 Jul;234(7):10512-22. https://doi.org/10.1002/jcp.27723
30. Pope AJ, Sands GB, Smaill BH, LeGrice IJ. Three-dimensional transmural organization of perimysial collagen in the heart. American Journal of Physiology-Heart and Circulatory Physiology. 2008 Sep;295(3):H1243-52. https://doi.org/10.1152/ajpheart.00484.2008
31. Quinn TA, Camelliti P, Rog-Zielinska EA, Siedlecka U, Poggioli T, O'Toole ET, Knöpfel T, Kohl P. Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proceedings of the National Academy of Sciences. 2016 Dec 20;113(51):14852-7. https://doi.org/10.1073/pnas.1611184114
32. Yáñez-Mó M, Siljander PR, Andreu Z, Bedina Zavec A, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles. 2015 Jan 1;4(1):27066. https://doi.org/10.3402/jev.v4.27066
33. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature cell biology. 2019 Jan;21(1):9-17. https://doi.org/10.1038/s41556-018-0250-9
34. Jesel L, Abbas M, Toti F, Cohen A, Arentz T, Morel O. Microparticles in atrial fibrillation: a link between cell activation or apoptosis, tissue remodelling and thrombogenicity. International journal of cardiology. 2013 Sep 30;168(2):660-9. https://doi.org/10.1016/j.ijcard.2013.03.031
35. Xiang K, Akram M, Elbossaty WF, Yang J, Fan C. Exosomes in atrial fibrillation: therapeutic potential and role as clinical biomarkers. Heart Failure Reviews. 2021 Jul 12:1-1. https://doi.org/10.1007/s10741-021-10142-5
36. Berezin AE, Berezin AA. Extracellular vesicles and thrombogenicity in atrial fibrillation. International Journal of Molecular Sciences. 2022 Feb 4;23(3):1774. https://doi.org/10.3390/ijms23031774
37. Shaihov-Teper O, Ram E, Ballan N, Brzezinski RY, Naftali-Shani N, Masoud R, Ziv T, Lewis N, Schary Y, Levin-Kotler LP, Volvovitch D. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation. 2021 Jun 22;143(25):2475-93. https://doi.org/10.1161/CIRCULATIONAHA.120.052009
38. Thulin Å, Lindbäck J, Granger CB, Wallentin L, Lind L, Siegbahn A. Extracellular vesicles in atrial fibrillation and stroke. Thrombosis Research. 2020 Sep 1;193:180-9. https://doi.org/10.1016/j.thromres.2020.07.029
39. Safavi-Naeini P, Rasekh A. Thromboembolism in atrial fibrillation: role of the left atrial appendage. Cardiac Electrophysiology Clinics. 2020 Mar 1;12(1):13-20. https://doi.org/10.1016/j.ccep.2019.11.003
40. Ramlawi B, Saleh WK, Edgerton J. The left atrial appendage: target for stroke reduction in atrial fibrillation. Methodist DeBakey Cardiovascular Journal. 2015 Apr;11(2):100. https://doi.org/10.14797/mdcj-11-2-100
41. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. Journal of Cell Biology. 2013 Feb 18;200(4):373-83. https://doi.org/10.1083/jcb.201211138
42. Mørk M, Andreasen JJ, Rasmussen LH, Lip GY, Pedersen S, Bæk R, Jørgensen MM, Kristensen SR. Elevated blood plasma levels of tissue factor-bearing extracellular vesicles in patients with atrial fibrillation. Thrombosis Research. 2019 Jan 1;173:141-50. https://doi.org/10.1016/j.thromres.2018.11.026
43. Kishima H, Mine T, Takahashi S, Ashida K, Ishihara M, Masuyama T. Morphologic remodeling of left atrial appendage in patients with atrial fibrillation. Heart Rhythm. 2016 Sep 1;13(9):1823-8. https://doi.org/10.1016/j.hrthm.2016.06.009
44. Gong S, Zhou J, Li B, Kang S, Ma X, Cai Y, Guo Y, Hu R, Zhang X. The association of left atrial appendage morphology to atrial fibrillation recurrence after radiofrequency ablation. Frontiers in Cardiovascular Medicine. 2021:852. https://doi.org/10.3389/fcvm.2021.677885
45. Negrotto SM, Lugo RM, Metawee M, Kanagasundram AN, Chidsey G, Baker MT, Michaud GF, Piana RN, Benjamin Shoemaker M, Ellis CR. Left atrial appendage morphology predicts the formation of left atrial appendage thrombus. Journal of Cardiovascular Electrophysiology. 2021 Apr;32(4):1044-52. https://doi.org/10.1111/jce.14922
46. Zhao J, Zhang Y, Yin Z, Zhu Y, Xin F, Zhang H, Po SS, Wang H. Impact of proinflammatory epicardial adipose tissue and differentially enhanced autonomic remodeling on human atrial fibrillation. The Journal of Thoracic and Cardiovascular Surgery. 2022 Mar 28. https://doi.org/10.1016/j.jtcvs.2022.03.013
47. Yamaguchi S, Otaki Y, Tamarappoo B, Yoshida J, Ikenaga H, Friedman J, Berman D, Dey D, Shiota T. The association between epicardial adipose tissue thickness around the right ventricular free wall evaluated by transthoracic echocardiography and left atrial appendage function. The International Journal of Cardiovascular Imaging. 2020 Apr;36:585-93. https://doi.org/10.1007/s10554-019-01748-w
48. Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999 Nov 1;82(5):547-54. http://dx.doi.org/10.1136/hrt.82.5.547
49. Oba K, Maeda M, Maimaituxun G, Yamaguchi S, Arasaki O, Fukuda D, Yagi S, Hirata Y, Nishio S, Iwase T, Takao S. Effect of the epicardial adipose tissue volume on the prevalence of paroxysmal and persistent atrial fibrillation. Circulation Journal. 2018 Jun 25;82(7):1778-87. https://doi.org/10.1253/circj.CJ-18-0021
50. Yang M, Bao W, Xu Z, Qin L, Zhang N, Yan F, Yang W. Association between epicardial adipose tissue and recurrence of atrial fibrillation after ablation: a propensity score-matched analysis. The International Journal of Cardiovascular Imaging. 2022 Aug;38(8):1865-72. https://doi.org/10.1007/s10554-022-02557-4
51. Yamada S, Kaneshiro T, Nodera M, Amami K, Nehashi T, Takeishi Y. Left atrial epicardial adipose tissue exacerbates electrical conduction disturbance in normal?weight patients undergoing pulmonary vein isolation for atrial fibrillation. Journal of Cardiovascular Electrophysiology. 2022 Dec 26. https://doi.org/10.1111/jce.15794
52. van der Heijden CA, Verheule S, Olsthoorn JR, Mihl C, Poulina L, van Kuijk SM, Heuts S, Maessen JG, Bidar E, Maesen B. Postoperative atrial fibrillation and atrial epicardial fat: Is there a link?. IJC Heart & Vasculature. 2022 Feb 21:100976. https://doi.org/10.1016/j.ijcha.2022.100976
53. Kogo H, Sezai A, Osaka S, Shiono M, Tanaka M. Does epicardial adipose tissue influence postoperative atrial fibrillation?. Annals of Thoracic and Cardiovascular Surgery. 2019;25(3):149-57. https://doi.org/10.5761/atcs.oa.18-00212
54. Gaeta M, Bandera F, Tassinari F, Lorenzo C, Cargnelutti M, Pelissero G, Malavazos AE, Ricci C. Is epicardial fat depot associated with atrial fibrillation? A systematic review and meta-analysis. EP Europace. 2017 May 1;19(5):747-52. https://doi.org/10.1093/europace/euw398
55. Wong CX, Sun MT, Odutayo A, Emdin CA, Mahajan R, Lau DH, Pathak RK, Wong DT, Selvanayagam JB, Sanders P, Clarke R. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circulation: Arrhythmia and Electrophysiology. 2016 Dec;9(12):e004378. https://doi.org/10.1161/CIRCEP.116.004378
56. Zhu W, Zhang H, Guo L, Hong K. Relationship between epicardial adipose tissue volume and atrial fibrillation. Herz. 2016 Aug 1;41(5):421. https://doi.org/10.1007/s00059-015-4387-z
57. Canpolat U, Aytemir K, Yorgun H, Asil S, Dural M, Özer N. The impact of echocardiographic epicardial fat thickness on outcomes of cryoballoon?based atrial fibrillation ablation. Echocardiography. 2016 Jun;33(6):821-9. https://doi.org/10.1111/echo.13193
58. Chen J, Mei Z, Yang Y, Dai C, Wang Y, Zeng R, Liu Q. Epicardial adipose tissue is associated with higher recurrence risk after catheter ablation in atrial fibrillation patients: a systematic review and meta-analysis. BMC Cardiovascular Disorders. 2022 Jun 11;22(1):264. https://doi.org/10.1186/s12872-022-02703-9
59. Nagashima K, Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune T, Kofune M, Mano H, Sonoda K, Hirayama A. Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circulation Journal. 2011;75(11):2559-65. https://doi.org/10.1253/circj.CJ-11-0554
60. Pokushalov E, Kozlov B, Romanov A, Strelnikov A, Bayramova S, Sergeevichev D, Bogachev-Prokophiev A, Zheleznev S, Shipulin V, Salakhutdinov N, Lomivorotov VV. Botulinum toxin injection in epicardial fat pads can prevent recurrences of atrial fibrillation after cardiac surgery: results of a randomized pilot study. Journal of the American College of Cardiology. 2014 Aug 12;64(6):628-9. https://doi.org/10.1016/j.jacc.2014.04.062
61. Sha R, Han W, Lin M, Zhong J. Is Epicardial Adipose Tissue Associated with Atrial Fibrillation Following Cardiac Surgery? A Systematic Review and Meta-Analysis. InThe Heart Surgery Forum 2021 Sep 13 (Vol. 24, No. 5, pp. E801-E807). https://doi.org/10.1532/hsf.3975
62. White CM, Sander S, Coleman CI, Gallagher R, Takata H, Humphrey C, Henyan N, Gillespie EL, Kluger J. Impact of epicardial anterior fat pad retention on postcardiothoracic surgery atrial fibrillation incidence: the AFIST-III Study. Journal of the American College of Cardiology. 2007 Jan 23;49(3):298-303. https://doi.org/10.1016/j.jacc.2006.10.033
63. Zhu YM, Xu HX, Lu Q, Huang YH, Jing HM, Wu X. Correlation between multi-slice spiral CT determined epicardial adipose tissue volume and atrial fibrillation. Zhonghua xin xue Guan Bing za zhi. 2019 Dec 1;47(12):969-73. https://doi.org/10.3760/cma.j.issn.0253-3758.2019.12.005
64. Werner S, Alzheimer C. Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine & growth factor reviews. 2006 Jun 1;17(3):157-71. https://doi.org/10.1016/j.cytogfr.2006.01.001
65. Wang Q, Min J, Jia L, Xi W, Gao Y, Diao Z, Zhang P, Wang S, Yang J, Wang L, Zhang Y. Human epicardial adipose tissue activin A expression predicts occurrence of postoperative atrial fibrillation in patients receiving cardiac surgery. Heart, Lung and Circulation. 2019 Nov 1;28(11):1697-705. https://doi.org/10.1016/j.hlc.2018.08.010
66. Park JS, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Echocardiographically measured epicardial fat predicts restenosis after coronary stenting. Scandinavian Cardiovascular Journal. 2013 Oct 1;47(5):297-302. https://doi.org/10.3109/14017431.2013.824604
67. Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clément K, Hatem SN. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. European heart journal. 2015 Apr 1;36(13):795-805. https://doi.org/10.1093/eurheartj/eht099
68. Boixel C, Fontaine V, Rücker-Martin C, Milliez P, Louedec L, Michel JB, Jacob MP, Hatem SN. Fibrosis of the left atria during progression of heart failure is associated with increased matrix metalloproteinases in the rat. Journal of the American College of Cardiology. 2003 Jul 16;42(2):336-44. https://doi.org/10.1016/S0735-1097(03)00578-3
69. Laxton RC, Hu Y, Duchene J, Zhang F, Zhang Z, Leung KY, Xiao Q, Scotland RS, Hodgkinson CP, Smith K, Willeit J. A role of matrix metalloproteinase-8 in atherosclerosis. Circulation research. 2009 Oct 23;105(9):921-9. https://doi.org/10.1161/CIRCRESAHA.109.200279
70. Haemers P, Hamdi H, Guedj K, Suffee N, Farahmand P, Popovic N, Claus P, LePrince P, Nicoletti A, Jalife J, Wolke C. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. European heart journal. 2017 Jan 1;38(1):53-61. https://doi.org/10.1093/eurheartj/ehv625
71. Gaborit B, Sengenes C, Ancel P, Jacquier A, Dutour-Meyer A. Role of epicardial adipose tissue in health and disease: a matter of fat?. Comprehensive physiology. 2017;7(3):1051-1082. https://doi.org/10.1002/cphy.c160034
72. Abe I, Teshima Y, Kondo H, Kaku H, Kira S, Ikebe Y, Saito S, Fukui A, Shinohara T, Yufu K, Nakagawa M. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart rhythm. 2018 Nov 1;15(11):1717-27. https://doi.org/10.1016/j.hrthm.2018.06.025
73. Suffee N, Moore-Morris T, Jagla B, Mougenot N, Dilanian G, Berthet M, Proukhnitzky J, Le Prince P, Tregouet DA, Puceat M, Hatem SN. Reactivation of the epicardium at the origin of myocardial fibro-fatty infiltration during the atrial cardiomyopathy. Circulation research. 2020 May 8;126(10):1330-42. https://doi.org/10.1161/CIRCRESAHA.119.316251
74. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, Finnie JW, Samuel CS, Royce SG, Twomey DJ, Thanigaimani S. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. Journal of the American College of Cardiology. 2015 Jul 7;66(1):1-1. https://doi.org/10.1016/j.jacc.2015.04.058
75. Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000 Jan 18;101(2):194-9. https://doi.org/10.1161/01.CIR.101.2.194
76. Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S. Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation. 2000 Nov 21;102(21):2619-28. https://doi.org/10.1161/01.CIR.102.21.2619
77. Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. Journal of cardiovascular electrophysiology. 2014 Sep;25(9):921-9. https://doi.org/10.1111/jce.12474
78. Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL, Crijns HJ. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circulation: Arrhythmia and Electrophysiology. 2010 Dec;3(6):606-15. https://doi.org/10.1161/CIRCEP.109.910125
79. de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 2010 Oct 26;122(17):1674-82. https://doi.org/10.1161/CIRCULATIONAHA.109.910901
80. Verheule S, Eckstein J, Linz D, Maesen B, Bidar E, Gharaviri A, Schotten U. Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Progress in biophysics and molecular biology. 2014 Aug 1;115(2-3):173-85. https://doi.org/10.1016/j.pbiomolbio.2014.07.007
81. Eckstein J, Zeemering S, Linz D, Maesen B, Verheule S, van Hunnik A, Crijns H, Allessie MA, Schotten U. Transmural conduction is the predominant mechanism of breakthrough during atrial fibrillation: evidence from simultaneous endo-epicardial high-density activation mapping. Circulation: Arrhythmia and Electrophysiology. 2013 Apr;6(2):334-41. https://doi.org/10.1161/CIRCEP.113.000342
82. Ausma J, Wijffels MC, Van Eys GJ, Koide M, Ramaekers F, Allessie M, Borgers M. Dedifferentiation of atrial cardiomyocytes as a result of chronic atrial fibrillation. The American journal of pathology. 1997 Oct;151(4):985.
83. Gambini E, Pesce M, Persico L, Bassetti B, Gambini A, Alamanni F, Agrifoglio M, Capogrossi MC, Pompilio G. Patient profile modulates cardiac c-kit+ progenitor cell availability and amplification potential. Translational Research. 2012 Nov 1;160(5):363-73. https://doi.org/10.1016/j.trsl.2012.05.009
84. Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, Twomey D, Gallagher C, Hendriks JM, Linz D, McEvoy RD. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. EP Europace. 2018 Dec 1;20(12):1929-35. https://doi.org/10.1093/europace/euy117
85. Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, Leong DP, Lau DH, Middeldorp ME, Roberts-Thomson KC, Wittert GA. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. Journal of the American College of Cardiology. 2011 Apr 26;57(17):1745-51. https://doi.org/10.1016/j.jacc.2010.11.045
86. Thanassoulis G, Massaro JM, O'Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circulation: Arrhythmia and Electrophysiology. 2010 Aug;3(4):345-50. https://doi.org/10.1161/CIRCEP.109.912055
87. Lee JJ, Yin X, Hoffmann U, Fox CS, Benjamin EJ. Relation of pericardial fat, intrathoracic fat, and abdominal visceral fat with incident atrial fibrillation (from the Framingham Heart Study). The American journal of cardiology. 2016 Nov 15;118(10):1486-92. https://doi.org/10.1016/j.amjcard.2016.08.011
88. Zheng ML, Du XP, Zhao L, Yang XC. Expression profile of circular RNAs in epicardial adipose tissue in heart failure. Chinese Medical Journal. 2020 Nov 5;133(21):2565-72. https://doi.org/10.1097/CM9.0000000000001056
89. Zheng H, Peng Y, Wang P, Su P, Zhao L. The integrative network of circRNA, miRNA and mRNA of epicardial adipose tissue in patients with atrial fibrillation. American Journal of Translational Research. 2022;14(9):6550-52.
90. Jiang S, Guo C, Zhang W, Che W, Zhang J, Zhuang S, Wang Y, Zhang Y, Liu B. The integrative regulatory network of circRNA, microRNA, and mRNA in atrial fibrillation. Frontiers in genetics. 2019 Jun 13;10:526. https://doi.org/10.3389/fgene.2019.00526
91. Wei F, Zhang X, Kuang X, Gao X, Wang J, Fan J. Integrated Analysis of circRNA-miRNA-mRNA-Mediated Network and Its Potential Function in Atrial Fibrillation. Frontiers in Cardiovascular Medicine. 2022;9.883205. https://doi.org/10.3389/fcvm.2022.883205
92. Wang Q, Shen H, Min J, Gao Y, Liu K, Xi W, Yang J, Yin L, Xu J, Xiao J, Wang Z. YKL-40 is highly expressed in the epicardial adipose tissue of patients with atrial fibrillation and associated with atrial fibrosis. Journal of Translational Medicine. 2018 Dec;16:1-9. https://doi.org/10.1186/s12967-018-1598-0
93. Sato T, Aizawa Y, Yuasa S, Fujita S, Ikeda Y, Okabe M. The effect of dapagliflozin treatment on epicardial adipose tissue volume and P-wave indices: an ad-hoc analysis of the previous randomized clinical trial. Journal of atherosclerosis and thrombosis. 2020 Dec 1;27(12):1348-58. https://doi.org/10.5551/jat.48009
94. Yang W, Shi H, Huang X, Ma Y, Guan B, Sun S, Yu Y, Luo J, Tian F, Cao J. Ideal cardiovascular health metrics and epicardial adipose tissue volume in a Northern Chinese population: a cross-sectional study. Annals of Translational Medicine. 2021 Jun;9(11):935. https://doi.org/10.21037/atm-21-1798
95. Y?lmaz AS, Çinier G, Ç?rako?lu ÖF, Çetin M. Epicardial adipose tissue predicted prolonged QTc interval in patients with arterial hypertension. Clinical and Experimental Hypertension. 2021 Apr 3;43(3):230-6. https://doi.org/10.1080/10641963.2020.1847131
