The COVID-19 Test Most of Us Need Now
Authors: Douglas F. Lake* and Alexa J. Roeder
Health Futures Center, School of Life Sciences, Arizona State University, Tempe, AZ, USA
*Correspondence to: Douglas F. Lake, Health Futures Center, Arizona State University, Phoenix, AZ 85054, USA; Tel: 602-543-4234, Email: Douglas.Lake@asu.edu
Received: July 01, 2022; Accepted: August 01, 2022; Published: August 07, 2022
Citation: Douglas F. Lake and Alexa J. Roeder (2022) The COVID-19 Test Most of Us Need Now 21st Century Pathology, Volume 2 (4): 124
Abstract
More than two years into the COVID-19 pandemic, a correlation of protection (probability of infection) is still unknown. Under certain circumstances, neither natural infection nor vaccination protects against re-infection which depends on many factors such as waning immunity and the emergence of escape variants. A rapid test that measures levels of neutralizing antibodies could provide a probability of infection that would allow immunocompromised individuals and their healthcare providers to make informed decisions about their healthcare and lifestyle, but only if the test reflects the viral sub-variant(s) in circulation at the time an individual takes the rapid test.
Keywords:
COVID-19; Neutralizing Antibody Rapid Test; Variants of concern
Introduction
As SARS-CoV-2 becomes endemic, it is not known what percentage of the population has been infected with ancestral or current sub-variants of SARS-CoV-2. However, the infection does not equate to protection, as viral infection may not induce protective immunity for two reasons. First, the mild infection may not elicit high levels of neutralizing antibodies (NAbs) [1]. In fact, NAbs were undetectable in over one-third of previously infected individuals [2,3]. Second, as the virus continues to mutate, variants arise that can escape neutralization, causing both symptomatic and asymptomatic infections which allow for continued viral transmission.
Prior to further discussion, it is important to distinguish infection from disease. Fortunately, the mRNA vaccines were ~95% effective at preventing hospitalization and severe disease [4,5]. The vaccines induce both Nabs [6] as well as anti-viral T cells [7,8]. However, as NAbs wane [9,10] -albeit faster for some than others?symptomatic re-infection occurs in many vaccine recipients [11,12] even before Omicron variants existed. Now that Omicron sub-variants are prevalent in the population, is there a test that can predict the probability of infection? The answer, of course, is ?it depends?.
Discussion
Early in the pandemic when the ancestral strain first detected in Wuhan was prevalent, we developed a rapid test13 that measures titers of antibodies that block the receptor binding domain (RBD) of the spike protein from binding to angiotensin-converting enzyme 2 (ACE2), the host cellular receptor for the virus [13]. These NAbs prevent the virus from infecting susceptible cells. Although there are additional regions on the SARS-CoV-2 spike protein that elicit Nabs [14], RBD is the principal neutralizing domain. NAbs apply selective pressure to the virus so that viral particles which escape neutralization will infect cells, replicate, and release progeny that may no longer be neutralized by vaccine-induced antibodies, leading to the evolution and spread of variants of concern.
As stated above, natural infection occurs on the virus? terms, and may not induce high titer NAbs (<1:50) in at least one-third of infections [2,3,15]. We confirmed this finding using our rapid NAb test on previously infected individuals. As shown in Figure 1 (baseline), the full spectrum of NAb titers was observed in previously infected individuals?from high NAb titers to titers that were not detectable, despite the previous infection. Interestingly, after previously infected individuals received their first dose of vaccine, over half showed high NAb titers (>1:640), suggesting immunologic priming during natural infection. Two weeks after a second mRNA vaccine dose, >77% of previously infected individuals had NAb titers at the highest level, 18% at NAb titers <1:320, ?1:160, and curiously, 4.6% had low NAb titers (<1:80, ?1:40). These findings suggest that hybrid immunity, even after 2 doses of mRNA vaccine, does not always induce high NAb titers.
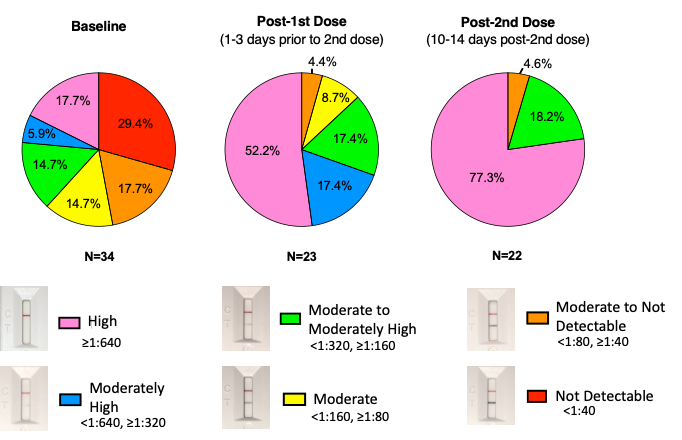
Figure 1: Neutralizing Antibody levels in previously infected individuals pre- and post-vaccination. Time points include baseline, post-1st dose, and post-2nd dose. Baseline refers to study participants prior to vaccination who were ?2 weeks and ?3 months post-infection. Post- 1st dose (1-3 days prior to 2nd dose) indicates either 21 or 28 days after the 2nd vaccine. All participants received either BNT162b2 or mRNA-1273 vaccines. Six levels of neutralization were calculated using test line density values based on the principle that neutralizing antibodies prevent RBD conjugated to gold beads from binding to ACE2 on a lateral flow strip, as previously described13. Percent neutralization was calculated as follows: 1-(Test Line Density/Limit of Detection)*100%. The limit of detection was determined to be a line density of 942,481. Percent neutralization ranges are follows: High (pink)=90-99%; Moderately high (blue)=80-89%; Moderate to Moderately high (green)=61-79%; Moderate (yellow)=36-60%; Moderate to Not Detectable (orange)=15-35%; Not detectable (Red)=?15% neutralization. Test line densities were quantified using an RDS-2500 lateral flow reader (Detekt Biomedical, Austin TX).
Many people have received 2 or 3 mRNA vaccine doses designed to protect against ancestral SARS-CoV-2. Since the emergence of Omicron in November/December 2021, hundreds of thousands of symptomatic breakthrough infections have occurred16. Since results of rapid at-home antigen tests are not reportable it has become difficult?if not impossible?to know what percentage of the population has had a breakthrough infection with an Omicron variant. Although currently, available mRNA vaccines provide some cross-protection against severe disease and hospitalization, they do not protect against Omicron variant infection, viral replication and, evidently, transmission [16,17].
Many immunosuppressed individuals or healthcare providers who work with immunocompromised patients do not know when immunity is waning, such that their probability of infection upon exposure is increased. Exposure and infection are particularly problematic for immunosuppressed persons as their cellular immunity is often impaired. Once infected due to inadequate levels of protective NAbs, the disease may occur and is more likely to be severe, leading to poor patient outcomes [18].
Conclusion
The COVID-19 pandemic has unfortunately demonstrated that individuals cannot rely on one another to practice protective behaviors such as wearing a mask and getting vaccinated. As a result, we have entered the age of communicable diseases in which individuals must protect themselves. Rapid tests that measure levels of NAbs from a finger-stick drop of blood could be used to monitor immunocompromised individuals for when a booster vaccine might be warranted. However, the rapid test must employ RBD or spike protein from the SARS-CoV-2 variant currently circulating in the population.
Otherwise, the test result would measure high titers of NAbs to vaccines based on the ancestral virus, but have limited bearing on protection from infection with the variant that is currently circulating. Similarly to manufacturers of the influenza vaccine, manufacturers of rapid NAb tests must monitor viral variants in the population, such that the results of their tests predict a probability of infection that reflects the current SARS- CoV-2 variant in circulation.
Authors? Contributions
Conceptualization, writing, reviewing and editing: DFL, AJR
Conflict of Interest
DFL is co-founder of Axim Biotech, a company that manufactures a rapid neutralizing antibody test. AJR has no potential conflict of interest relevant to this article.
References
1. Legros V, Denolly S, Vogrig M, Boson B, Siret E, Rigaill J, Pillet S, Grattard F, Gonzalo S, Verhoeven P, Allatif O. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cellular & molecular immunology. 2021 Feb;18(2):318-27. https://doi.org/10.1038/s41423-020-00588-2
2. Juno JA, Tan HX, Lee WS, Reynaldi A, Kelly HG, Wragg K, Esterbauer R, Kent HE, Batten CJ, Mordant FL, Gherardin NA. Immunogenic profile of SARS-CoV-2 spike in individuals recovered from COVID-19. medRxiv. 2020 Jan 1. https://doi.org/10.1101/2020.05.17.20104869
3. Robbiani DF, Gaebler C, Muecksch F, Lorenzi JC, Wang Z, Cho A, Agudelo M, Barnes CO, Gazumyan A, Finkin S, Hägglöf T. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020 Aug;584(7821):437-42. https://doi.org/10.1101/2020.05.13.092619
4. Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416. https://doi.org/10.1056/NEJMoa2035389
5. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Marc GP, Moreira ED, Zerbini C, Bailey R. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England journal of medicine. 2020 Dec 10. https://doi.org/10.1056/NEJMoa2034577
6. Lake DF, Roeder AJ, Gonzalez-Moa MJ, et al. Third COVID-19 Vaccine Dose Boosts Neutralising Antibodies in Poor Responders. Infectious Diseases (except HIV/AIDS); 2021. https://doi.org/10.1101/2021.11.30.21266716
7. Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, Lundgreen KA, Reynaldi A, Khoury DS, Pattekar A, Gouma S. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021 Dec 3;374(6572):abm0829. https://doi.org/10.1126/science.abm0829
8. Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, Baxter AE, Herati RS, Oldridge DA, Gouma S, Hicks P. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021 Sep 14;54(9):2133-42. https://doi.org/10.1016/j.immuni.2021.08.001
9. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, Rubin C. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. New England Journal of Medicine. 2021 Dec 9;385(24):e84. https://doi.org/10.1056/NEJMoa2114583
10. Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, Milo R, Alroy-Preis S, Ash N, Huppert A. Waning immunity after the BNT162b2 vaccine in Israel. New England Journal of Medicine. 2021 Dec 9;385(24):e85. https://doi.org/10.1056/NEJMoa2114228
11. Brehm TT, Pfefferle S, von Possel R, Kobbe R, Nörz D, Schmiedel S, Grundhoff A, Olearo F, Emmerich P, Robitaille A, Günther T. SARS-CoV-2 reinfection in a healthcare worker despite the presence of detectable neutralizing antibodies. Viruses. 2021 Apr 12;13(4):661. https://doi.org/10.3390/v13040661
12. Cohen JI, Burbelo PD. Reinfection with SARS-CoV-2: implications for vaccines. Clinical Infectious Diseases. 2021 Dec 1;73(11):e4223-8. https://doi.org/10.1093/cid/ciaa1866
13. Lake DF, Roeder AJ, Kaleta E, Jasbi P, Pfeffer K, Koelbela C, Periasamy S, Kuzmina N, Bukreyev A, Grys TE, Wu L. Development of a rapid point-of-care test that measures neutralizing antibodies to SARS-CoV-2. Journal of Clinical Virology. 2021 Dec 1;145:105024. https://doi.org/10.1016/j.jcv.2021.105024
14. Kumar S, Chandele A, Sharma A. Current status of therapeutic monoclonal antibodies against SARS-CoV-2. PLoS pathogens. 2021 Sep 3;17(9):e1009885. https://doi.org/10.1371/journal.ppat.1009885
15. Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, Ling Y, Zhang Y, Xun J, Lu L, Jiang S. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. MedRxiv. 2020 Jan 1. https://doi.org/10.1101/2020.03.30.20047365
16. Kuhlmann C, Mayer CK, Claassen M, Maponga T, Burgers WA, Keeton R, Riou C, Sutherland AD, Suliman T, Shaw ML, Preiser W. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. The Lancet. 2022 Feb 12;399(10325):625-6. https://doi.org/10.1016/S0140-6736(22)00090-3
17. Hachmann NP, Miller J, Collier AR, Ventura JD, Yu J, Rowe M, Bondzie EA, Powers O, Surve N, Hall K, Barouch DH. Neutralization escape by SARS-CoV-2 Omicron subvariants BA. 2.12. 1, BA. 4, and BA. 5. New England Journal of Medicine. 2022 Jul 7;387(1):86-8. https://doi.org/10.1056/NEJMc2206576
18. Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clinical Infectious Diseases. 2021 Jan 15;72(2):340-50. https://doi.org/10.1093/cid/ciaa863
