Short Commentary - A Story of Trichomonads for Pathologists
Author: Christophe DUBOUCHER*
Department of Pathology, Intercommunal Hospital of Poissy / St-Germain-en-Laye, France
*Correspondence to: Christophe Duboucher, Department of Pathology, Intercommunal Hospital of Poissy / St-Germain-en-Laye, 29 Raymond Queneau Street, 92500 Rueil Malmaison, France; E-mail: christophe.duboucher@yahoo.com
ORCID: https://orcid.org/0000-0002-5698-3742
Received: November 07, 2021; Accepted: December 24, 2021; Published: December 28, 2021
Citation: Duboucher C (2021) Short Commentary - A Story of Trichomonads for Pathologists, 21st Century Pathol, Volume 1 (1): 107
Abstract
For many clinicians and pathologists, Trichomonas vaginalis is the only species of trichomonad that they have encountered during their studies and their practice. Nevertheless, other species of the order Trichomonadida inhabit humans. Furthermore, zoonotic infections and new human species of Trichomonads have recently been described. However, infection of humans by species of Trichomonads outside their natural site is also unknown by physicians. Nevertheless, pathologists may regularly observe this protozoan but – without recognizing it –, in deep tissues, especially in lungs, in the course of Pneumocystosis or acute respiratory disease syndrome. It is therefore important that this parasite becomes more widely acknowledged and recognized. Its pathogenic role requires detailed assessment.
Keywords:
Trichomonads; Trichomoniasis; Parasitic diseases; Zoonotic diseases; Pneumocystis pneumonia; ARDS; SARS-CoV-2
Introduction
In a letter to the editor, recently published in the Journal of Infection [1], we informed clinicians and parasitologists about frequent superimposed infection of the lung by Trichomonads during acute respiratory disease syndrome (ARDS), including the ongoing SARS-CoV-2 pandemic. We have here the opportunity to present to readers of 21st Century Pathology mainly pathologists the observations that allowed us to describe these superimposed infections. Discussion is made of the reasons that explain why this description has not been identified earlier. Questions that arise are debated and recent developments are presented.
The Story
In the 2000s, we found numerous amoeboid forms of Trichomonads in association with aggregates of Pneumocystis jirovecii (P. jirovecii) in the bronchoalveolar lavage fluid (BALF) of an HIV+ patient. Following small subunit (SSU) rRNA gene amplification and sequencing, Trichomonas vaginalis (T. vaginalis) was identified [2]. Some years later, another case of co-infection by a trichomonad and P. jirovecii was observed. In this second case, the trichomonad was identified as Tritrichomonas foetus (T. foetus) [3]. T. foetus is known in cattle, swine, and cats, but is very rarely found in humans. At this time, it was not known if we were dealing with a strain coming from an animal, indicating true zoonosis, or an undescribed strain adapted to humans.
These two observations of pulmonary co-infection by P. jirovecii and Trichomonads led us to examine in retrospect MGG stained slides of BALF archived in our laboratory. We found, by optical examination, the presence of Trichomonads in 60% of BALFs of patients suffering from P. jirovecii pneumonia (PJP) (66 cases). In addition, the frequency and abundance of Trichomonads increase to a frequency of 100% in cases with the highest pneumocystis burden (15 cases) [4].
Thus, the disease entitled "PJP" could be considered perhaps provocatively as a co-infection of P. jirovecii and Trichomonads.
A few years later, finding amoeboid forms of Trichomonads in a BALF from a patient without Pneumocystosis or HIV infection, but diseased from ARDS, led us to review slides from the intensive care unit of the same establishment. By optical examination, Trichomonads were detected in 33% of the BALFs of patients presenting ARDS (17 patients; only two with PJP). When considering the delay between admission and BALF sample, an incidence of 65% was observed beyond day 5 [5]. We conclude that superinfection by Trichomonads may be considered a potential event during ARDS, and perhaps also provocatively an event that will arrive sooner or later.
Several questions arise from these observations:
I - What Defines an Amoeboid Form?
This is the first question that must be asked.
In alveolar lumens, Trichomonads adhere to epithelial cells. They evolve from a flagellated form to an amoeboid form. They develop pseudopods and lose their flagella. Thus, they lose their distinctive feature and look like anonymous cells. In particular, they can mimic human cells (Figure 1).
Figure 1: Microphotographs from BALFs of Pneumocystis pneumonia and ARDS (MGG stain, x1000). Trichomonads are indicated by arrows. Figure 1A-B: Trichomonads of small size that cannot be interpreted as human cells. Figure 1C: Trichomonad of medium size that can be erroneously considered as human cells. Figure 1D: Large amoeboid Trichomonads that cannot be interpreted without awareness of this amazing form. Figure 1E: Large binucleated cell regularly observed. Figure 1F: Multinucleated amoeboid trichomonad sometimes found.
This amoeboid transformation is well documented for T. vaginalis in the genital tract [6]. A dense network of actin-rich microfilaments is observed by electron microscopy along the adhesion zone [7].
In BALFs, amoeboid Trichomonads exhibit salient polymorphism. The smallest cells show an elliptic nucleus. The size of their nucleus, compared with those of lymphocytes human cells with the smallest nucleus, indicates a non-human cell. However, binucleated big cells with two large and round nuclei are also observed. They could be interpreted as dystrophic human cells. Furthermore, transition forms are always found, from smallest to biggest cells, indicating a common nature. Finally, these nuclear characteristics are not those of human cells and a parasite a protist must be invoked as an explanation.
II ? How can we Confirm that these Unidentified Cells are the Trichomonads Found by Molecular Biology?
It is a crucial question for pathologists who are observers and who want to identify what they see.
The protist was identified as T. vaginalis in the first case and T. foetus in the second case after SSU rRNA gene amplification or by ITS1-5.8S rRNA-ITS2 region gene amplification, respectively, followed by sequencing.
Nevertheless, if molecular identification confirms that the parasite is present in the sample, it does not prove that the unidentified cells seen under the microscope are the parasite.
As previously noted, transformation to amoeboid forms involves a reduction of microtubular structures (flagella and axostyle-pelta complex). Such structures were not detected by light microscopy on BALF cytospin slides stained with MGG, or by immuno-cytochemistry using a specific anti-tubulin monoclonal antibody (Figure 2A-B).
Consequently, four additional methods were used to confirm that these cells were Trichomonads:
- Immunohistochemistry with an anti-trichomonas serum (Figure 2C).
- In situ hybridization with an Alu-targeted probe. Alu are repetitive elements found in mammalian genomes, especially in primates. Lack of hybridization confirms the non-human nature of protozoan cells (Figure 3A)
- In situ hybridization with a probe designed to label rRNA of the family Trichomonadidae (Figure 3B).
- In situ hybridization with a probe designed to label the repeated transposable elements Tvmar1 of T. vaginalis (Figure 3C).
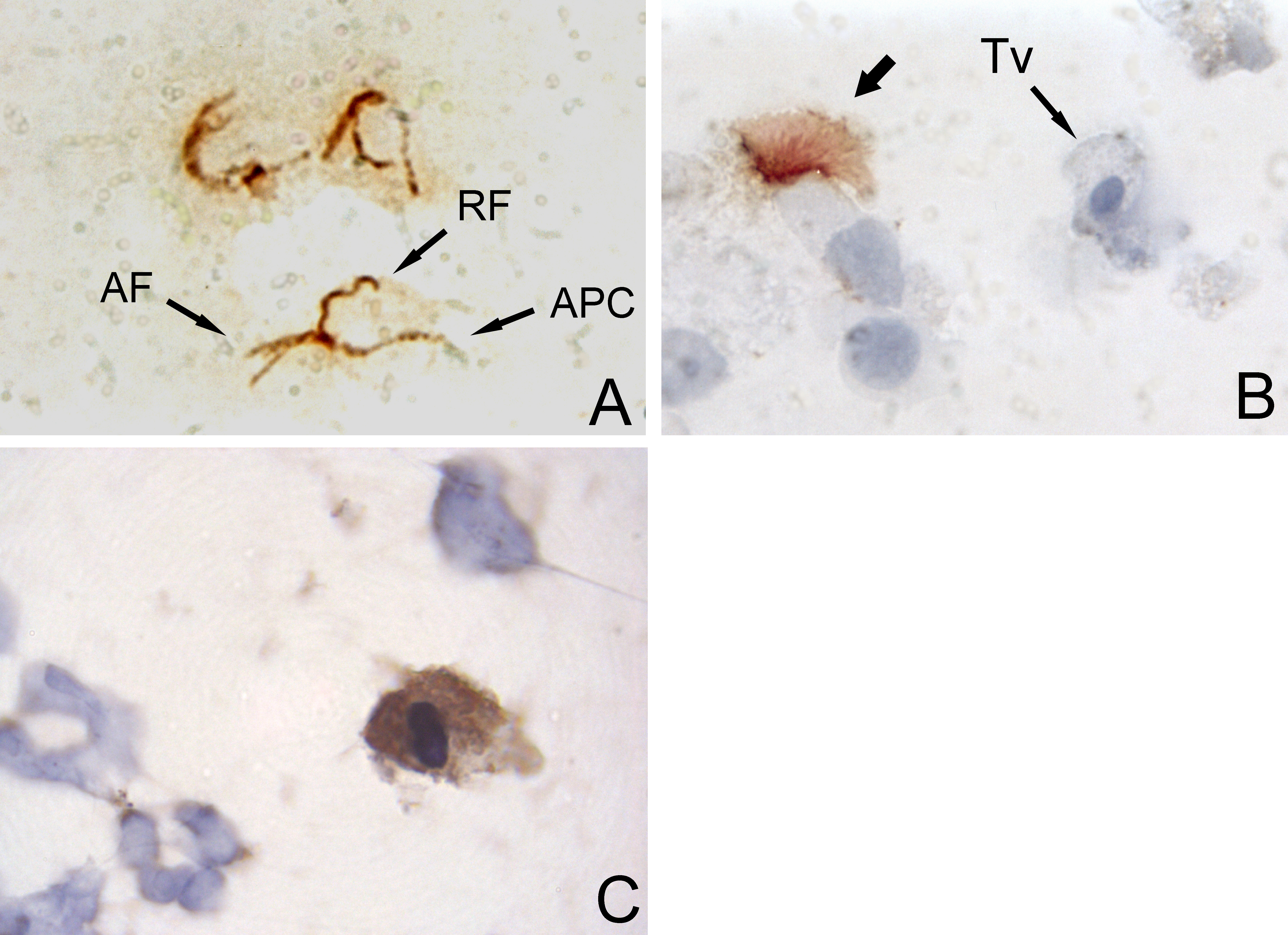
Figure 2: Set of micrographs of immunocytochemistry with DAB chromogene.
Figure 2A & 2B: Labeling of Trichomonas vaginalis cells with an anti-tubulin anti-serum (x1000). A. Labeling of microtubular structures of T. vaginalis cells in culture (positive control). The anterior flagella (AF), recurrent flagellum (RF), and axostyle-pelta complex (APC), are easily recognizable. B. In the amoeboid trichomonad of a BALF (Tv), no microtubular structure is recognized by the same antibody, whereas the cilia of bronchial cells are labelled (thick arrow). The protozoan was identified herein as T. vaginalis [2].
Figure reproduced from the publication by Duboucher C, et al. (2003) [2]. With permission from Elsevier.
Figure 2C: Labeling of a trichomonad using anti-trichomonas antibody (x1000).
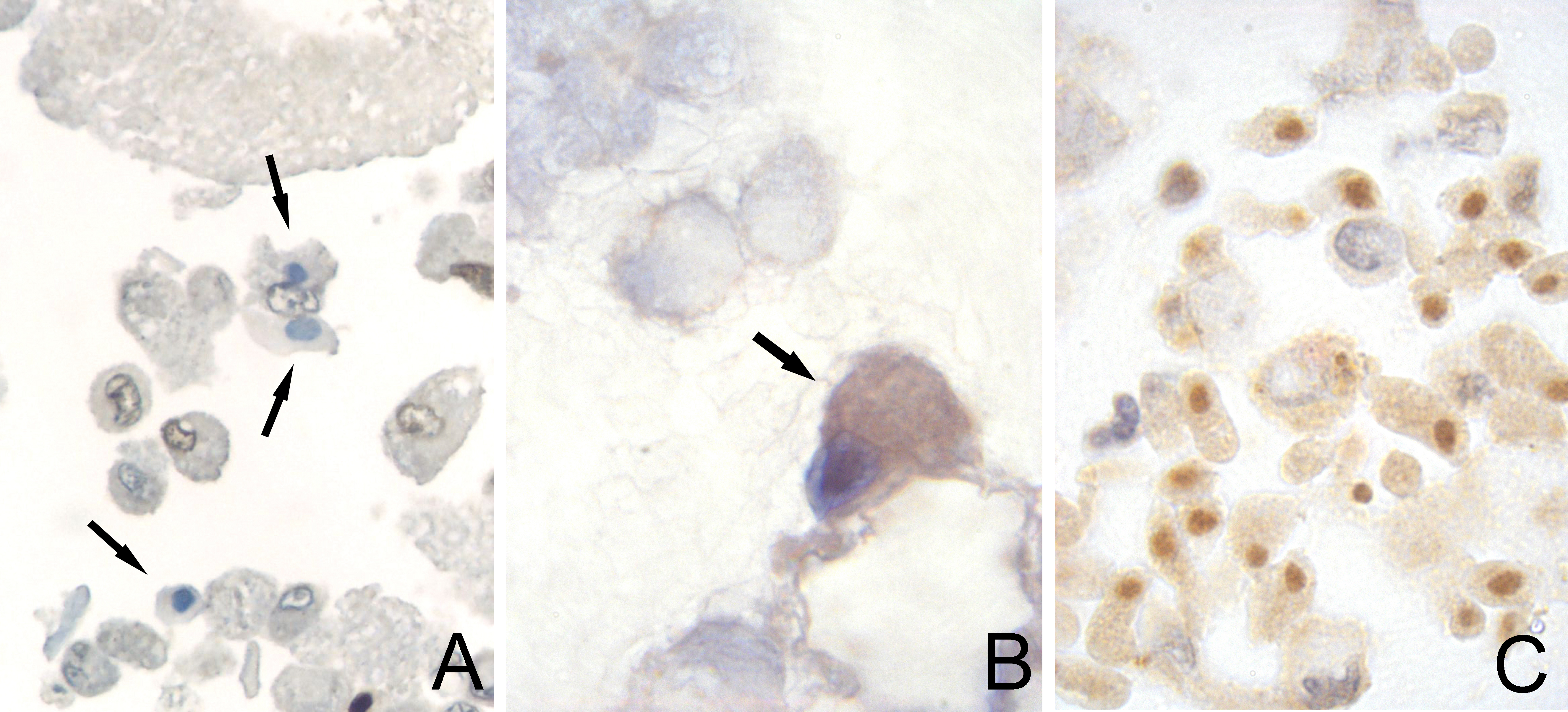
Figure 3: Set of micrographs of in situ hybridization (ISH) with DAB chromogene.Figure 3A: ISH using Alu-targeted probe in a cell pellet from a BALF (x400). Three parasitic cells with blue nucleus (arrows) are recognized among macrophages. Only macrophages nuclei are labelled.
Figure 3B: ISH using a probe targeting SSU-rRNA in a cell pellet from a BALF (x1000). A parasitic cells is labelled (arrow).
Figure 3C: ISH using Tvmar1-targeted probe in a cell pellet of cultivated T. vaginalis mixed with human cells from pleural cavity (x400). Tvmar1 is a specific target for the species T. vaginalis.
III - What is the Reason for the Development of Trichomonads in Alveolar Lumens?
Several factors are proposed, none of which are mutually exclusive.
Optical observation of cytospins of BALFs of PJP demonstrated tight contacts between Trichomonads and pneumocystis aggregates or isolated trophozoites. One hypothesis is that Trichomonads fed on pneumocystis. The ability of T. vaginalis for phagocytosis and degradation of fungi is known [6].
A second hypothesis is related to the fact that Trichomonads are microaerophilic organisms. They tolerate only low levels of oxygen [8]. Reduction of ventilation generated by pneumocystis pullulating may create the oxygen-poor environment required for trichomonad development. This second hypothesis is supported by the observation of Trichomonads in BALFs of ARDS. In ARDS, local hypoxic conditions could be a critical factor favoring the multiplication of Trichomonads, because the alveolar lumens are filled with fibrin and cellular debris. Thus, immunosuppression is not an essential condition for the development of Trichomonads, because this was apparent in superimposed infection during ARDS, without evident immunosuppression.
IV ? Is there a Deleterious Action of Amoeboid Trichomonads in BALFs?
Amoeboid transformation of T. vaginalis in the genital tract is well known, in vitro and in vivo. When transformed, T. vaginalis adheres strongly to host cells. Interaction and pathogenic effects are more clearly understood [9,10].
The deleterious action of Trichomonads during PJP or ARDS remains unproven. Nevertheless, the amoeboid transformation of Trichomonads removed from alveolar lumens argues for their aggressiveness.
In PJP, it could be postulated that the ending of the triggering factor that is pneumocystis multiplication ? is sufficient to cure the trichomonad superimposed infection.
Nevertheless, in ARDS of any origin including SARS-CoV-2 or in severe forms of PJP when diffuse alveolar damage is combined, it remains possible that Trichomonads may delay healing and impose the limit to recovery. The potentially deleterious action during ARDS needs to be evaluated by clinical trials.
V - Why has this Observation not been Made Previously?
It remains somewhat surprising that frequent superimposed infection of lungs by Trichomonads during PJP and ARDS has not been identified earlier, and there must be a reason for this.
In BALFs, Trichomonads lose their distinctive feature and look like anonymous cells or mimic human cells. Consequently, on slides colored using MGG, amoeboid Trichomonads do not display their familiar appearance to parasitologists or cytopathologists.
Until our first cases of Pneumocystosis linked to AIDS, numerous BALFs have been analyzed, and no publications by cytopathologists or parasitologists include the keyword "Trichomonads".
Among numerous publications on Pneumocystosis, few aimed to explore cell composition, and all but one did not mention the presence of unidentified cells. Nevertheless, in one publication, nonidentified cells are mentioned [11].
The authors, Jacobs et al., drew to cells nonidentified regularly found in BALFs during their analyses of cell composition in Pneumocystosis, which they named NICs. They tried to identify these NICs, but without result.
These NICs are presented in their publication (Figure 4). A comparison of their NICs with amoeboid Trichomonads that we present (Figure 1) confirms that Jacobs JA, et al. (2001) have clearly described cells that are now recognized as amoeboid Trichomonads.
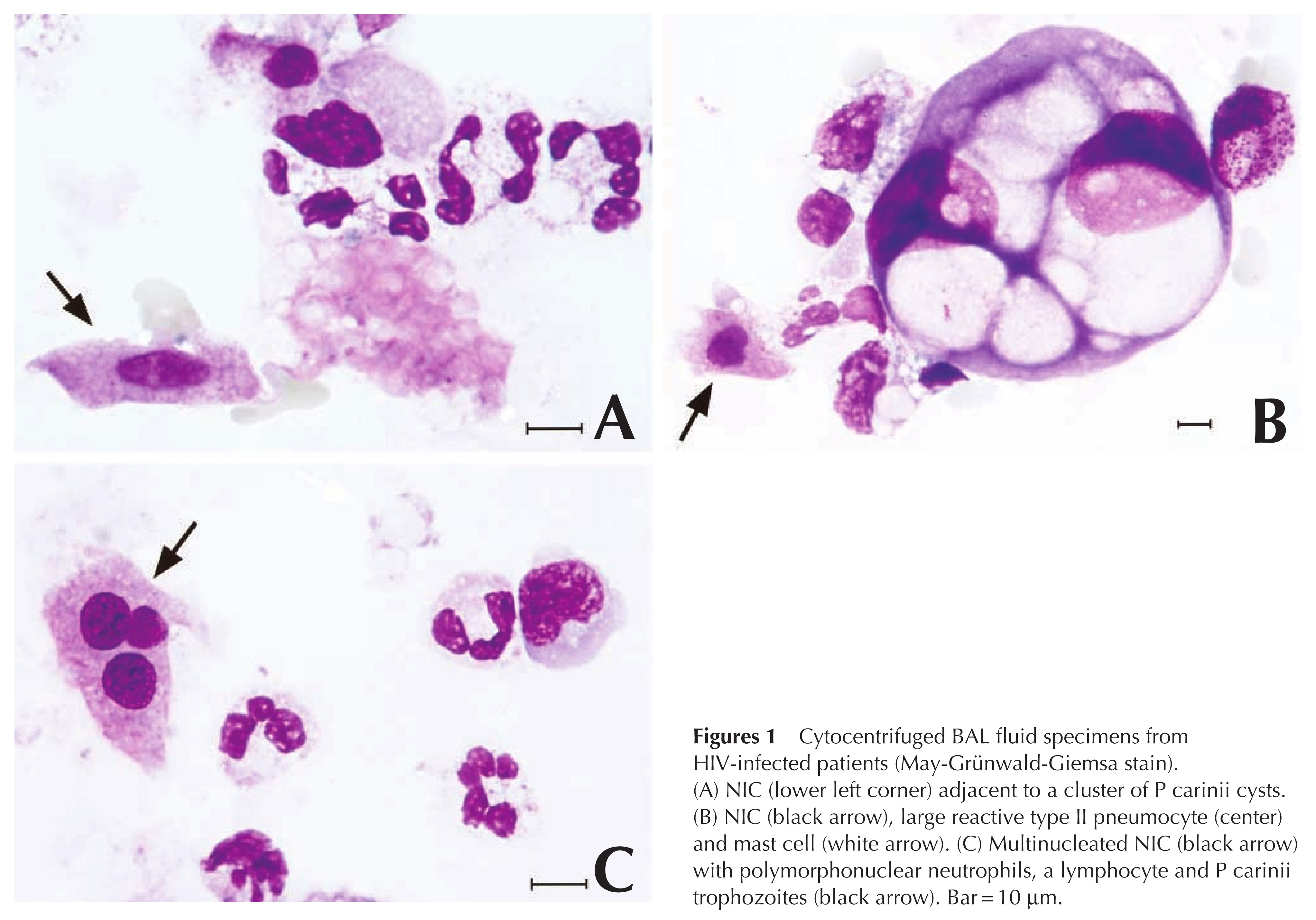 Figure 4: Microphotographs from the publication by Jacobs JA, et al. (2001) [11]. The comparing with Figure 1 clearly shows that these cells are the same as the cells presented in Figure 1. Figure reproduced from the publication by Jacobs JA, et al. (2001) [11]. With permission from Karger.
Figure 4: Microphotographs from the publication by Jacobs JA, et al. (2001) [11]. The comparing with Figure 1 clearly shows that these cells are the same as the cells presented in Figure 1. Figure reproduced from the publication by Jacobs JA, et al. (2001) [11]. With permission from Karger.Other observers analyzing BALF of PJP or ARDS have presumably seen these NICs and have questioned their nature. Observers who are familiar with human cells pathologists rather the parasitologists ? have probably looked carefully at these cells, and may have deduced that they could be parasitic cells. Perhaps, they asked a parasitologists. But it is vain because, for a parasitologists, who observes Trichomonads in fresh specimens, these cells do not appear as parasites, especially a trichomonad which by definition bears flagella. A trichomonad is a "flagellated" in the traditional taxonomic position. Finally, without an answer from a parasitologists, the pathologist cannot identify these NICs.
Perhaps, other observers have judiciously believed them to be a parasite, but could not ask the question of its identity in a publication, because generally publications with questions, but no answers, are not acceptable for publication.
Jacobs JA, et al. (2001) were doubly lucky. First, they screened and tried to name all the cells. They isolated a "cell orphan" named "NIC" [11]. In studies by others, cell composition was analyzed and observers counted the cells that they recognized. This procedure is quite different from that of Jacobs JA, et al. (2001) because following this procedure results in identification only of cells that the observers recognize. The publication by Fleury J, et al. (1985) [12] is an example of these publications, sound but in which no "bizarre cell" were noticed. Secondly, Jacobs JA, et al. (2001) had the advantage of presenting results, namely, the cytological composition in BALFs from PJP, in addition to their question on NICs. The presentation of these results probably facilitated publication.
Nevertheless, Jacobs JA, et al. (2001) did not ask the question of the origin of these cells, that is, human or non-human. The presence of amoeboid Trichomonads too small to be a human cell should have revealed their parasitic nature (Figure 1 A-B). Furthermore, the authors, believing that these NICs were human, did not include any discussion on the possibility of a parasitic nature of their NICs.
VI ? What is the Origin of Trichomonads Found in the Lungs of Humans?
Some species identified by us or others in lungs are well known in humans, including Trichomonas tenax (T. tenax), T. vaginalis, or Pentatrichomonas hominis (P. hominis). T. tenax, often present in the mouth, is sometimes also found in lung abscess or in pyopneumothorax [13]. T. vaginalis has been found in lungs in rare cases, mainly in infants [14]. P. hominis living in the large intestine has been found in only one empyema [15]. Thus, the human species of Trichomonads are not limited to their traditional site.
Two genera known in animals have also been found in humans: Tritrichomonas and Tetratrichomonas. Tritrichomonas foetus (T. foetus) is found in animals, and its taxonomy remains debated [16]. T. foetus has been found in humans on rare occasions [17], and a zoonotic nature of the disease may be favoured.
Tetratrichomonas is a genus found in birds, primates, cattle, and tortoises. Tetratrichomonas strains have been isolated by Kutisova K, et al. (2005) [18] from bronchi and oral cavities of patients suffering various pulmonary diseases. These strains exhibit a very close genetic relationship with a common avian species, Tetratrichomonas gallinarum (T. gallinarum). But a zoonotic origin has not been proven because attempts to transmit Tetratrichomonas of human origin to birds failed. Thus, the existence of a human-host-adapted T. gallinarum-like strain cannot be excluded.
More recently, a new species of Tetratrichomonas, presently unknown in animals, has been identified in France in a patient suffering from pyopneumothorax [19]. A few years later, in Mexico, this same species, identified using ITS1-5.8S rRNA-ITS2 sequences, has been found in two patients presenting pyopneumothorax [20]. A species belonging to humans was considered, and the name Tetratrichomonas empyemagena (T. empyemagena) was proposed. Finally, in France again, T. empyemagena was found in a patient presenting Pneumocystosis without pleural lesion [21], reinforcing the view of a species belonging to humans, and probably an inhabitant of lungs finally emerged from obscurity.
VII ? What are the Challenges for Pathologists in the 21st Century?
Although the deleterious action of Trichomonads has not yet been admitted by all apart from T. vaginalis, detection of Trichomonads should not be neglected.
Several challenges can be identified:
- Assessment of the aggressiveness of Trichomonads in lungs during PJP, ARDS, and other diseases.
- Involvement in prostate carcinogenesis by T. vaginalis [22], or in gastrointestinal carcinogenesis by P. hominis [23].
- Identification of infections by zoonotic Trichomonads [24].
- Discovery of new human species of Trichomonads.
PCR is very efficient in detecting Trichomonads and in their identification in fresh specimens. Nevertheless, pathologists deal with fixed specimens unsuitable for PCR. Furthermore, visualization of the parasite is needed to interpret pathologic lesions. There is no stain that labels the parasite in histologic specimens. Sera has been developed for Immuno-cyto-chemistry but does not work in specimens fixed in formalin.
Labeling by in situ hybridization (ISH) is feasible.
1°) SSU rRNA may be targeted by probes, designed for the family Trichomonadidae (Figure 4B) or for different species within this family. Mostegl MM, et al. (2010) [25] have published the whole procedure that can be applied successfully.
2°) The Whole sequencing of T. vaginalis, achieved in 2004 [26], has surprisingly shown numerous sequences repeated. Among them, the repeated transposable element Tvmar1 [27] is a perfect target (Figure 4C), for example for pathologists analyzing prostate specimens by ISH. The whole sequencing of T. foetus has also been available since 2017 [28] and revealed the same surabondance of repeated sequences as T. vaginalis.Conclusion
Young pathologists of the 21st century must be aware that discoveries by observation through an optical microscope remain possible. They will gain in watching with naive eyes what others have neglected, and in making observations with an open mind (Figure 5).
Young pathologists must be aware that the interpretation of what we see is sometimes altered by what we have learned, and they must question what is sententiously taught. Moreover, they must be ready to face unbelievers [29].
Young pathologists must enlarge their knowledge to encompass other field?s closeness of human pathology, and which are often neglected including zoology, veterinary pathology, and history of medicine.
Lopez-Escamilla E, et al. (2013) [20] said in the conclusion of their report: "It is relevant to alert parasitologists [and pathologists also!] to the potential occurrence of Trichomonads, alone or as co-infecting agents, in pulmonary disease milieus and to underline the importance of direct microscopic examination of fresh samples, as well as the usefulness of molecular methods for species identification because we are certain that new cases of pulmonary trichomonosis caused by this new species [T. empyemagena] will appear".
Parasitologists and cytopathologists need to be convinced by pictures. Microphotographs from different cases of deep trichomoniasis are visible at https://www.trichomoniasis-pathology.org/
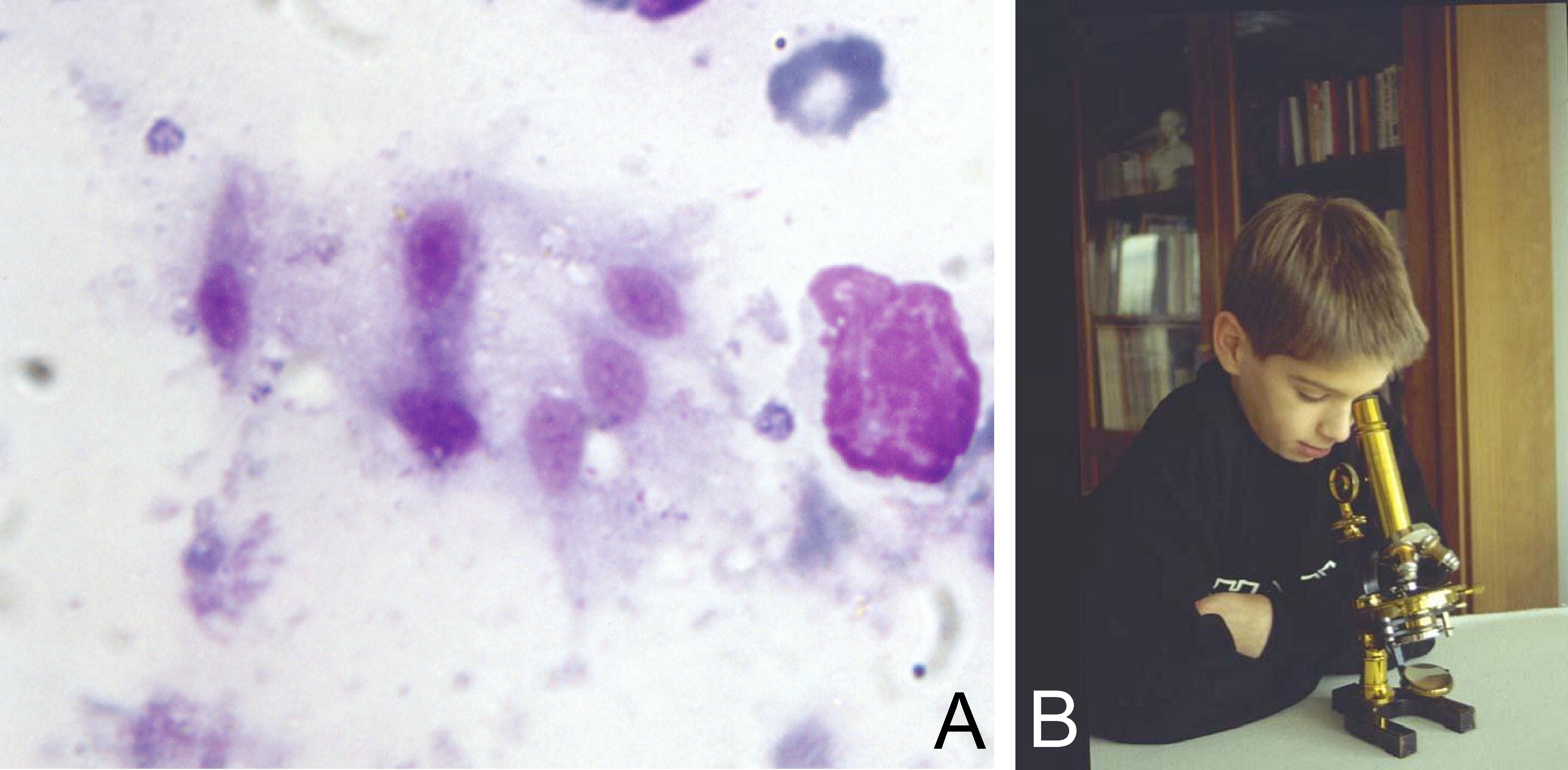 Figure 5: Figure 5A: Six amoeboid Trichomonads observed on a touch print of lymph node removed from an 82-year-old woman [20] (MGG stain, x1000). How many other cases have been overlooked?
Figure 5: Figure 5A: Six amoeboid Trichomonads observed on a touch print of lymph node removed from an 82-year-old woman [20] (MGG stain, x1000). How many other cases have been overlooked?
Figure reproduced from the publication by Duboucher C, et al. (2000) [30]. With permission from Elsevier.
Figure 5B: It is not the magnification of our microscope that will help us. It is observation with naive eyes that will help us.Funding Sources
No funding source is to be declared.
Declaration of Competing Interest
No competing interest is to be declared.
Financial Disclosure
The author declares no relevant financial or non-financial relationships to disclose.
Acknowledgement
None.
References
1. Duboucher C (2021) SARS-CoV-2 and superimposed infection by Trichomonads. J Infect 82: e22-e23.
https://doi.org/10.1016/j.jinf.2020.11.038
2. Duboucher C, Noel C, Durand-Joly I, et al. (2003) Pulmonary coinfection by Trichomonas vaginalis and Pneumocystis sp. as a novel manifestation of AIDS. Hum Pathol 34: 508-511.
https://doi.org/10.1016/s0046-8177(03)00088-1
3. Duboucher C, Caby S, Dufernez F, et al. (2006) Molecular identification of Tritrichomonas foetus-like organisms as coinfecting agents of human Pneumocystis pneumonia. J Clin Microbiol 44: 1165-1168.
https://doi.org/10.1128/JCM.44.3.1165-1168.2006
4. Duboucher C, Gerbod D, Noel C, et al. (2005) Frequency of Trichomonads as coinfecting agents in Pneumocystis pneumonia. Acta Cytol 49: 273-277. https://doi.org/10.1159/000326149
5. Duboucher C, Barbier C, Beltramini A, et al. (2007) Pulmonary superinfection by Trichomonads in the course of acute respiratory distress syndrome. Lung 185: 295-301.
https://doi.org/10.1007/s00408-007-9022-1
6. Hirt RP (2013) Trichomonas vaginalis virulence factors: an integrative overview. Sex Transm Infect 89: 439-443. https://doi.org/10.1136/sextrans-2013-051105
7. Nielsen MH, Nielsen R (1975) Electron microscopy of Trichomonas vaginalis Donne: interaction with vaginal epithelium in human trichomoniasis. Acta Pathol Microbiol Scand B 83: 305-320.
https://doi.org/10.1111/j.1699-0463.1975.tb00107.x
8. Lamien-Meda A, Leitsch D (2020) Identification of the NADH-oxidase gene in Trichomonas vaginalis. Parasitol Res 119: 683-686. https://doi.org/10.1007/s00436-019-06572-8
9. Ryan CM, de Miguel N, Johnson PJ (2011) Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem 51: 161-175. https://doi.org/10.1042/bse0510161
10. Leitsch D (2021) Recent advances in the molecular biology of the protist parasite Trichomonas vaginalis. Fac Rev 10: 26. https://doi.org/10.12703/r/10-26
11. Jacobs JA, Dieleman MM, Cornelissen EI, et al. (2001) Bronchoalveolar lavage fluid cytology in patients with Pneumocystis carinii pneumonia. Acta Cytol 45: 317-326.
https://doi.org/10.1159/000327625
12. Fleury J, Escudier E, Pocholle MJ, et al. (1985) Cell population obtained by bronchoalveolar lavage in Pneumocystis carinii pneumonitis. Acta Cytol 29: 721-726.
13. Wu Y, Ye Y, Yang Y, et al. (2021) Pyopneumothorax from coinfection by Trichomonas tenax and Geotrichum capitatum in a child from China: a case report. BMC Infect Dis 21: 842.
https://doi.org/10.1186/s12879-021-06539-0
14. Hamilton H, Pontiff KL, Bolton M, et al. (2018) Trichomonas vaginalis Brain Abscess in a Neonate. Clin Infect Dis 66: 604-607. https://doi.org/10.1093/cid/cix908
15. Jongwutiwes S, Silachamroon U, Putaporntip C (2000) Pentatrichomonas hominis in empyema thoracis. Trans R Soc Trop Med Hyg 94: 185-186. https://doi.org/10.1016/s0035-9203(00)90270-0
16. D?browska J, Keller I, Karamon J, et al. (2020) Whole genome sequencing of a feline strain of Tritrichomonas foetus reveals massive genetic differences to bovine and porcine isolates. Int J Parasitol 50: 227-233. https://doi.org/10.1016/j.ijpara.2019.12.007
17. Yao C (2012) Opportunistic human infections caused by Tritrichomonas species: A Mini-Review. Clin Microbiol Newsl 34: 127-134. https://doi.org/10.1016/j.clinmicnews.2012.07.004
18. Kutisova K, Kulda J, Cepicka I, et al. (2005) Tetratrichomonads from the oral cavity and respiratory tract of humans. Parasitology 131: 309-319. https://doi.org/10.1017/s0031182005008000
19. Mantini C, Souppart L, Noel C, et al. (2009) Molecular characterization of a new Tetratrichomonas species in a patient with empyema. J Clin Microbiol 47: 2336-2339.
https://doi.org/10.1128/JCM.00353-09
20. Lopez-Escamilla E, Sanchez-Aguillon F, Alatorre-Fernandez CP, et al. (2013) New Tetratrichomonas species in two patients with pleural empyema. J Clin Microbiol 51: 3143-3146.
https://doi.org/10.1128/JCM.01056-13
21. Mille S, Toubas D, Foudrinier F, et al. (2020) Prévalence de détection des Trichomonadines associées à Pneumocystis jirovecii. Étude rétrospective au CHU de Reims [Prevalence of detection of trichomonadines associated with Pneumocystis jirovecii. Retrospective study at the Reims University Hospital]. Poster session presented at: One health – De l'animal à l'homme en parasitologie-Mycologie; 2020, May 22–24; Tours, France.
22. Sutcliffe S, Neace C, Magnuson NS, et al. (2012) Trichomonosis, a common curable STI, and prostate carcinogenesis-a proposed molecular mechanism. PLoS Pathog 8: e1002801.
https://doi.org/10.1371/journal.ppat.1002801
23. Zhang N, Zhang H, Yu Y, et al. (2019) High prevalence of Pentatrichomonas hominis infection in gastrointestinal cancer patients. Parasit Vectors 12: 423. https://doi.org/10.1186/s13071-019-3684-4
24. Maritz JM, Land KM, Carlton JM, et al. (2014) What is the importance of zoonotic Trichomonads for human health? Trends Parasitol 30: 333-341. https://doi.org/10.1016/j.pt.2014.05.005
25. Mostegl MM, Richter B, Nedorost N, et al. (2010) Design and validation of an oligonucleotide probe for the detection of protozoa from the order Trichomonadida using chromogenic in situ hybridization. Vet Parasitol 171: 1-6. https://doi.org/10.1016/j.vetpar.2010.03.022
26. Carlton JM, Hirt RP, Silva JC, et al. (2007) Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315: 207-212. https://doi.org/10.1126/science.1132894
27. Bradic M, Warring SD, Low V, et al. (2014) The Tc1/mariner transposable element family shapes genetic variation and gene expression in the protist Trichomonas vaginalis. Mob DNA 5: 12.
https://doi.org/10.1186/1759-8753-5-12
28. Benchimol M, de Almeida LGP, Vasconcelos AT, et al. (2017) Draft Genome Sequence of Tritrichomonas foetus Strain K. Genome Announc 5: e00195-17.
https://doi.org/10.1128/genomeA.00195-17
29. Duboucher C, Boggia R, Morel G, et al. (2007) Pneumocystis pneumonia: immunosuppression, Pneumocystis jirovecii...and the third man. Nat Rev Microbiol 5: 967.
https://doi.org/10.1038/nrmicro1621-c1
30. Duboucher C, Farto-Bensasson F, Cheron M, et al. (2000) Lymph node infection by Trichomonas tenax: report of a case with co-infection by Mycobacterium tuberculosis. Hum Pathol 31: 1317-1321.
