Overview of Carcinoid Heart Disease
Ezra A. Amsterdam*, Chengyue Jin, Shahad Al Chalaby, Sandhya Venugopal, and Gagan Singh
*Division of Cardiovascular Medicine, Department of Internal Medicine, University of California (Davis) School of Medicine, Sacramento, CA
*Corresponding author: Ezra A. Amsterdam, MD, Distinguished Professor, Cardiology and Internal Medicine University of California (Davis) School of Medicine, Stockton Blvd, Sacramento, CA, USA; E-mail: eaamsterdam@ucdavis.edu
Received: 26 May 2023; Accepted: 01 June 2023; Published: 04 June 2023
Copyright: © 2023 Amsterdam EA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Sun Y, Peng C, Zhang S (2023) Unexpected Roles of Soluble α-Synuclein Post-translational Modifications in Pathological α-Synuclein Amplification, 21st Century Pathology, Volume 3 (4): 149
Citation: Amsterdam EA, Jin C, Al Chalaby S, Venugopal S, Singh G. Overview of Carcinoid Heart Disease, 21st Century Cardiol. 2023 Apr; 3(3):136
Abstract
Carcinoid heart disease (CHD) is largely a syndrome of the right-sided cardiac valves resulting from the effects of neurotransmitters released by a carcinoid tumor. It is rare, potentially life-threatening, and is a manifestation of vasoactive substances such as serotonin produced by the tumor. These substances promote fibroblast growth that causes valvular fibrosis, functional impairment, regurgitation and stenosis, and ultimately right ventricular failure and marked fluid retention. Left heart structures are rarely involved because serotonin is destroyed in the pulmonary circulation. Treatment focuses primarily on control of the underlying carcinoid disease, heart failure, and surgical replacement of the affected right heart valves.
Keywords:
Carcinoid heart disease; Cardiac valves; Neurotransmitters; Carcinoid tumor
Pathophysiology and Pathology
Progression from carcinoid syndrome to CHD is frequent. A principal mechanism of CHD is serotonin-induced proliferation of fibroblasts, which results in extensive accumulation of scar tissue on the tricuspid and pulmonary valves and ventricular walls [1,4,5]. The valves are thickened, mobility is impaired (Figure 1), and tricuspid and pulmonary regurgitation and stenosis ensue followed by heart failure [1-3]. Serotonin can also augment cardiac sympathetic activity causing arrhythmias and coronary spasm. The tumor is usually located in the gut, but small NETs may not be detected. Left heart structures are protected by enzymatic degradation of serotonin in the lungs, resulting in a low frequency of left-sided CHD (<10%) [1-3,6]. CHD of left heart structures arise from a right-to-left shunt via a patent foramen ovale enabling venous return to bypass the lungs [1-3,6]. The importance of serotonin in the development of CHD is supported by markedly elevated levels of circulating serotonin in those with the disease [1,4,5]. Additionally, urinary 5-hydroxy indole acetic acid (5-HIAA), the major metabolite of serotonin, is also increased in patients with CHD [1,3-5].
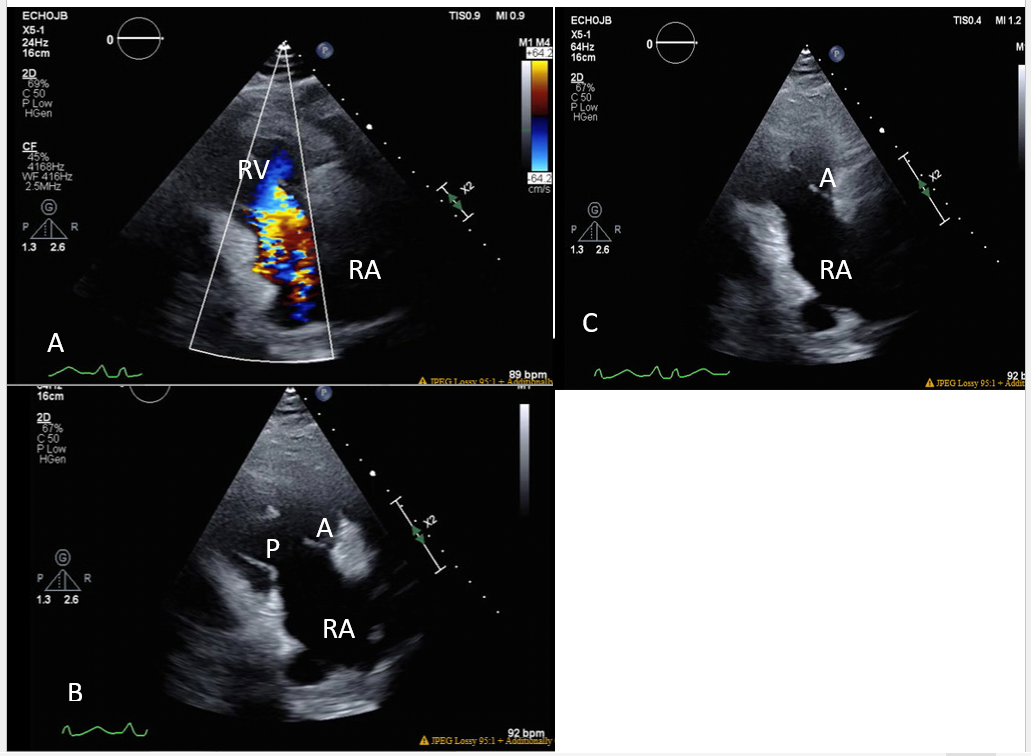
Figure 1: Two-dimensional echocardiogram, right ventricular inflow view. A) The color flow image demonstrates severe tricuspid valve regurgitation. RA-right atrium, RV-right ventricle. B) Diastolic phase. The tricuspid leaflets are thickened and in the open position. RA-right atrium, A-anterior leaflet, P-posterior leaflet. C) Systolic phase. The tricuspid leaflets remain in the open position during systole. The posterior leaflet is obscured. A-anterior leaflet.
Presentation and Diagnosis
Symptom onset in CHD is usually between ages 50 and 70, and the disease inexorably progresses to moderate-severe right ventricular failure and substantial fluid retention [1-3,7]. Examination reveals the regurgitant and stenotic murmurs of the right-sided cardiac valves, of which tricuspid regurgitation is typically most prominent; this murmur is ubiquitous in CHD patients. Electrocardiography is nonspecific and chest radiography may demonstrate right-sided chamber enlargement. Echocardiography is the principal imaging modality and discloses the characteristic valvular lesions of CHD [1,3,5,8]. Computed tomography and cardiac magnetic resonance can provide further structural and functional details.
Management and Prognosis
In untreated patients with CHD, median survival is 11 months and mortality is usually attributable to heart failure [1-3]. Medical management includes treatment of heart failure, debulking of hepatic metastases, and use of somatostatin analogues to inhibit tumor secretion of vasoactive agents. These measures have had limited success on symptoms and no effect on the valvular disease. Surgical replacement of the involved tricuspid and/or pulmonary valves has been the most effective treatment and high operative risk has improved at specialized centers. In a retrospective study of 195 CHD patients with NYHA class 3 or 4 heart failure, Mayo Clinic reported a decrease in surgical mortality from 20% (surgery before 1990) to 6% (surgery in this century) [9]. In this cohort, all patients received tricuspid valve replacement, many had additional procedures such as concomitant pulmonary valve replacement, and seven patients underwent quadruple valve replacement. Ten-year survival was 24% (18% to 32%), and 75% of patients had improved symptoms. One patient was alive > 19 years after surgery. Two important investigations may further advance this challenging aspect of CHD. The TEER trial (tricuspid transcatheter edge-to-edge repair) recently demonstrated the feasibility of catheter-based repair for severe tricuspid regurgitation [10]. TEER is a prospective randomized trial of patients with severe tricuspid regurgitation and cardiac failure. The etiologies of tricuspid regurgitation were not noted in the report. Additionally, the potential role of catheter-based tricuspid valve replacement is currently under investigation in a single arm registry of the TRISCEND II Pivotal Clinical Trial (NCT04482062). The long-term durability of the prosthetic valve tissue in this trial and its impact on survival is currently uncertain in patients with CHD.
Conclusion
Carcinoid heart disease is caused by a neuroendocrine tumor that secretes elevated levels of neurotransmitters such as serotonin. These agents have fibroblastic properties that promote plaque-like scarring of primarily right heart valves, impairing function with resultant tricuspid and pulmonary valve regurgitation and stenosis. The carcinoid process is progressive and results in right heart failure and marked fluid retention. Survival is poor in untreated patients, and medical therapies have limited value. Left heart structures are typically spared by the degradation of serotonin in the lungs. The therapy of choice has been surgical removal and replacement of the diseased valves, but operative risk is high. Catheter based methods to repair or replace the tricuspid valve are currently under investigation.
References
1. Davar J, Connolly HM, Caplin ME, Pavel M, Zacks J, Bhattacharyya S, Cuthbertson DJ, Dobson R, Grozinsky-Glasberg S, Steeds RP, Dreyfus G. Diagnosing and managing carcinoid heart disease in patients with neuroendocrine tumors: an expert statement. Journal of the American College of Cardiology. 2017 Mar 14;69(10):1288-304. https://doi.org/10.1016/j.jacc.2016.12.030
2. Pellikka PA, Tajik AJ, Khandheria BK, Seward JB, Callahan JA, Pitot HC, Kvols LK. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993 Apr;87(4):1188-96. https://doi.org/10.1161/01.CIR.87.4.1188
3. Jin C, Sharma AN, Thevakumar B, Majid M, Al Chalaby S, Takahashi N, Tanious A, Arockiam AD, Beri N, Amsterdam EA. Carcinoid heart disease: pathophysiology, pathology, clinical manifestations, and management. Cardiology. 2021;146(1):65-73. https://doi.org/10.1159/000507847
4. Lundin L, Norheim I, Landelius J, Oberg K, Theodorsson-Norheim E. Carcinoid heart disease: relationship of circulating vasoactive substances to ultrasound-detectable cardiac abnormalities. Circulation. 1988 Feb;77(2):264-9. https://doi.org/10.1161/01.CIR.77.2.264
5. Denney WD, Kemp WE, Anthony LB, Oates JA, Byrd BF. Echocardiographic and biochemical evaluation of the development and progression of carcinoid heart disease. Journal of the American College of Cardiology. 1998 Oct;32(4):1017-22. https://doi.org/10.1016/s0735-1097(98)00354-4
6. Mansencal N, Mitry E, Forissier JF, Martin F, Redheuil A, Lepère C, Farcot JC, Joseph T, Lacombe P, Rougier P, Dubourg O. Assessment of patent foramen ovale in carcinoid heart disease. American heart journal. 2006 May 1;151(5):1129-e1. https://doi.org/10.1016/j.ahj.2006.02.019
7. Bhattacharyya S, Davar J, Dreyfus G, Caplin ME. Carcinoid heart disease. Circulation. 2007 Dec 11;116(24):2860-5. https://doi.org/10.1161/CIRCULATIONAHA.107.701367
8. Bhattacharyya S, Toumpanakis C, Burke M, Taylor AM, Caplin ME, Davar J. Features of carcinoid heart disease identified by 2-and 3-dimensional echocardiography and cardiac MRI. Circulation: Cardiovascular Imaging. 2010 Jan;3(1):103-11. https://doi.org/10.1161/CIRCIMAGING.109.886846
9. Connolly HM, Schaff HV, Abel MD, Rubin J, Askew JW, Li Z, Inda JJ, Luis SA, Nishimura RA, Pellikka PA. Early and late outcomes of surgical treatment in carcinoid heart disease. Journal of the American College of Cardiology. 2015 Nov 17;66(20):2189-96. https://doi.org/10.1016/j.jacc.2015.09.014
10. Sorajja P, Whisenant B, Hamid N, Naik H, Makkar R, Tadros P, Price MJ, Singh G, Fam N, Kar S, Schwartz JG. Transcatheter repair for patients with tricuspid regurgitation. New England Journal of Medicine. 2023 Mar 4;388:1833-1842. https://doi.org/10.1056/NEJMoa2300525
