How do Soluble Guanylate Cyclase and other Hemoproteins Selectively Bind Gaseous Ligands
Author: Gang Wu*
Hematology-Oncology Division, Department of Internal Medicine, The University of Texas – McGovern Medical School, USA
*Corresponding author: Hematology-Oncology Division, Department of Internal Medicine, the University of Texas – McGovern Medical School, 6431 Fannin Street, Houston, TX 77030, USA; E-mail: gang.wu@uth.tmc.edu
Received: 14 April 2023; Accepted: 25 April 2023; Published: 28 April 2023
Citation: Wu G, (2023) How do Soluble Guanylate Cyclase and other Hemoproteins Selectively Bind Gaseous Ligands 21st Century Cardiol, Volume 3 (2): 133
Abstract
Nitric oxide (NO), carbon monoxide (CO), and oxygen (O2) are important physiological messengers whose concentrations vary in a remarkable range, from nM of [NO] to hundreds of µM of [O2]. Soluble guanylate cyclase (sGC), the only authentic heme-based acceptor for NO in mammals can selectively bind picomolar level of NO while excludes O2-binding. This exceptional selectivity of sGC can be interpreted by the “sliding scale rule”, which identifies factors that govern the gaseous ligand selectivity in hemoproteins.
Keywords:
Sliding scale rule; Soluble guanylate cyclase; Gaseous ligand selectivity; Heme sensor/binding proteins
Introduction
Small diatomic gas molecules carbon monoxide (CO), nitric oxide (NO), and oxygen (O2) exist universally in our environment and are generated in living organisms by various pathways. They often play important messenger roles to trigger various physiological responses. Many heme sensor proteins exist in living organisms to selectively bind these gaseous messengers to initiate downstream response cascades. Due to the huge difference in the concentrations of these molecules, [NO] from nM to several μM, [CO] in nM range, and [O2] may up to hundreds of μM, a heme sensor protein such as soluble guanylate cyclase (sGC) which is the only authentic mammalian heme-based acceptor for NO, faces the challenge to selectively bind NO against the enormous background of O2.
In human, the binding of NO to sGC boosts its guanosine 3',5'-cyclic monophosphate (cGMP) synthesis activity by several hundred-fold, leading to relaxation of vascular smooth muscle cells, calcium sequestration, reduction of platelet aggregation, and many other physiological responses. sGC is a heterodimer of an α and a β subunits, each containing a Heme NO-Oxygen binding domain (H-NOX), a Per-Arnt-Sim domain (PAS), a coiled-coil domain (CC), and a catalytic domain (CAT). Only the β H-NOX domain contains a heme prosthetic group. NO binding to the heme triggers a conformational change transducing from the H-NOX domains to the CAT domains through the PAS and CC domains, leading to the binding of substrate GTP and cGMP synthesis [1]. Intriguingly, sGC is capable binding pM NO but excludes O2-binding even at 1.3 mM O2 (equilibrated with 1 atm air). This phenomenal selectivity of sGC can be interpreted well by the recently proposed “sliding scale rule” [2].
“Sliding Scale Rule”
The molecular basis of gaseous ligand binding in sGC and other heme sensor proteins is a heme singly ligated to a neutral histidine. Small heme model compound ligated to a neutral imidazole possesses intrinsic selectivity for NO, CO, and O2 with large affinity ratios KD(CO)/KD(NO) ≈ KD(O2)/KD(CO) ≈ 103 ? 104. This significant intrinsic gaseous ligand selectivity is borne out in many hemoproteins which bind to a heme though a neutral histidine ligand, such as sGC. If we plot the logarithms of gaseous ligand affinity in a hemoprotein versus the ligand type, the log KD’s fall on an approximately straight line (Figure 1A). Moreover, such lines in different hemoproteins parallel each other due to the similar ratios of ligand affinity. This graphic rendering of gaseous ligand selectivity is coined the “sliding scale rule”. An immediate application of this “rule” is the estimation of a gaseous ligand affinity which is difficult to measure experimentally. For example, KD(O2) for sGC which cannot be measured experimentally is estimated to be ~1.3 M by drawing a line through its measured KD(NO) or KD(CO) (Figure 1A).
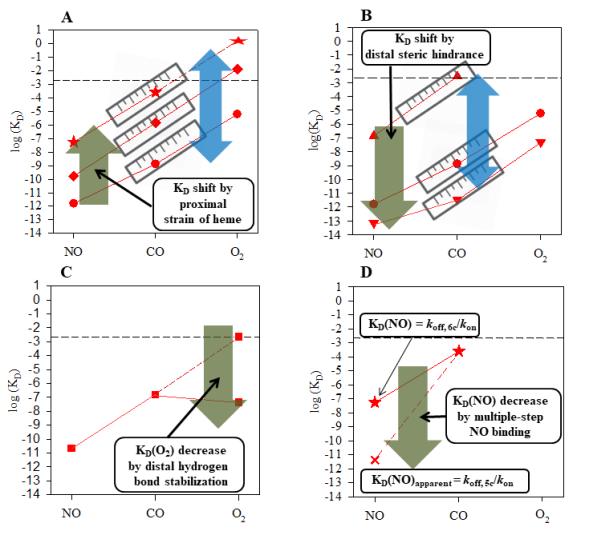
Figure 1: Figure 1: Factors that govern gaseous ligand selectivity in hemoproteins. The “sliding scale rule” is illustrated using binding data from sGC, two bacterial H-NOXs (sGC analogues), cytochrome c’ (Cyt c’), and heme model compound PP(1-MeIm). The logarithms of experimentally measured KD values of hemoproteins and model compounds to NO, CO, and O2 (red symbols) are plotted versus ligand type. Circle, PP(1-MeIm); star, sGC; up triangle, Cyt c’; down triangle, Leucine 16 to alanine Cyt c’ mutant (L16A Cyt c’); diamond, H-NOX from Nostoc sp. (Ns H-HOX); square, Cs H-NOX. The horizontal dashed line in each panel represents.
The 103 ? 104-fold intrinsic gaseous ligand selectivity of a neutral histidine-ligated heme may be inadequate for a hemoprotein due to the much wider concentration range of these molecules in living organisms. The gaseous ligand selectivity of hemeproteins is regulated tremendously by mechanisms which either uniformly adjust the affinities of all three gaseous ligands or selectively for either O2 or NO.
Structural elements in hemoproteins apply proximal strain to heme or obstruct the access to heme though steric hindrance on the distal side, these effects uniformly shift the intrinsic affinity for all three gaseous ligands but maintain the affinity ratios. The proximal strain of heme modulates the bond strength between the heme iron atom and the proximal histidine ligand, attuning the intrinsic affinity of heme for all three ligands. In sGC, the proximal strain comes from the domain-domain and subunit-subunit interactions and profoundly decreases the affinities
of all three gases, leading to the exclusion of O2-binding in sGC (Figure 1A). On the other hand, some hemoproteins have a bulky amino acid sidechain(s) on the distal side of the heme, impeding gaseous ligand binding by steric hindrance. Distal steric hindrance also indiscriminately lowers the affinity in a hemoprotein for all three gaseous ligands. Through proximal strain and/or distal steric hindrance, hemoproteins dramatically expand their range of affinity for NO, CO, and O2 by about 8 ? 9 orders of magnitude.
Hemoproteins may specifically enhance the affinity for O2 or NO. The hemoproteins that function as sensor or transporter or O2 boost their affinities for O2 through a distal hydrogen bond donor(s), which forms hydrogen bond to heme bound O2 and stabilizes the oxyferrous complex, leading to a dramatically enhanced affinity for O2 (Figure 1C). A good example is the H-NOX protein from Caldanaerobacter subterraneus (Cs H-NOX), a bacterial analogue of sGC, in which distal tyrosine 140 stabilizes the oxyferrous complex, leading to strong Cs H-OX affinity for O2 (Figure 1C). The high affinities of hemoglobin and myoglobin for O2 are due to a distal histidine residue that stabilizes the oxyferrous heme.
Due to the proximal strain of heme, the intrinsic affinity of sGC for NO is weakened to only 54 nM. However, the histidine to sGC heme dissociates quickly after NO binding, leading to nearly irreversible association of NO with heme and large conformational change. This multiple steps in NO binding boosts sGC’s apparent affinity for NO to pM range (Figure 1D) and introduces the significant conformational change necessary for cGMP production. Multiple-step NO binding is also observed in several bacterial analogues of sGC.
Conclusion
The “sliding scale rule” identifies five key factors govern the gaseous ligand selectivity of hemoproteins: a neutral histidine ligand to heme, the proximal strain of heme, distal steric hindrance, distal hydrogen bond donor(s), and multiple-step in NO binding. The “sliding scale rule” provides a unified model for gaseous ligand selectivity in hemoproteins.
References
1. Kang Y, Liu R, Wu JX, Chen L. Structural insights into the mechanism of human soluble guanylate cyclase. Nature. 2019 Oct 10;574(7777):206-10. https://doi.org/10.1038/s41586-019-1584-6
2. Wu G, Martin E, Berka V, Liu W, Garcin ED, Tsai AL. A new paradigm for gaseous ligand selectivity of hemoproteins highlighted by soluble guanylate cyclase. Journal of inorganic biochemistry. 2021 Jan 1;214:111267. https://doi.org/10.1016/j.jinorgbio.2020.111267
