High Yield Review of the Uses of Cardiac CT in Heart Failure
Authors: Spencer S Kitchin1*, Venkat Sanjay Manubolu2, Mathew J Budoff3
1Department of Internal Medicine, Harbor-UCLA Medical Center, Torrance, California, United States
2Department of Internal Medicine, Los Angeles Biomedical Research Institute, Torrance, California, United States
*Correspondence to: Spencer Kitchin, Harbor-UCLA Medical Center, 1000 W Carson St, Box #400 Torrance, CA, 90502, United States of America; E-mail: skitchin@dhs.lacounty.gov
Received: 09 October 2022; Accepted: 25 October 2022; Published: 28 October 2022
ORCID:
Spencer Kitchin: 0000-0002-6985-3437
Matthew J Budoff: 0000-0002-9616-1946
Authors Contribution
Conceptualization: SSK. Image curation: VSM. Project Administration: MJB. Writing original draft: SSK. Writing review & editing: SSK, VSM, MJB
Conflict of Interest
If there are any conflicts of interest, authors should disclose them in the manuscript. If there are no any conflicts of interest, authors should describe following sentence. ?No potential conflict of interest relevant to this article was reported?.
Citation: Spencer S Kitchin, Venkat Sanjay Manubolu and Mathew J Budoff (2022) High Yield Review of the Uses of Cardiac CT in Heart Failure 21st Century Cardiol, Volume 2 (5): 125
Abstract
Heart failure (HF) is a widespread disease. These patients need evaluation into the etiology of the heart failure, and require personalized cardiovascular care tailored to their heart disease (coronary artery disease, structural, electrophysiology). One of the ways these patients can be managed is with cardiac computed tomography (CT), which is a versatile tool that can be used to rule out ischemic etiology of heart failure, assess hemodynamic consequences of coronary artery disease, evaluate the myocardium, and assist in various procedural interventions. Cardiac CT has seen huge advances in technology allowing for novel uses over the last decade. Recently, the authors wrote a detailed narrative review on the uses of cardiac CT in heart failure to review these uses [1]. This commentary will address high yield topics on how cardiac CT can be integrated into the clinical care of patients with HF in accordance with major society guidelines, as well as future uses of this modality.
Keywords:
Cardiac computed tomography; Heart failure; Valvular disease; Coronary artery disease
Introduction
Heart failure (HF) is a widespread disease with significant morbidity and mortality attached to it. These patients require investigation into the etiology and complications of heart failure so that they can be treated with customized care targeted towards any combination of coronary artery disease (CAD), structural disease, and arrythmias. One of the ways these patients can be managed is with cardiac computed tomography (CT), which is a versatile tool by which physicians can glean significant information about their patients. Cardiac CT is a rapidly evolving field, and providers may feel overwhelmed and underprepared to use this modality. Recently, the authors wrote a detailed narrative review on the uses of cardiac CT in heart failure to remedy this [1]. This commentary will address high yield topics on how cardiac CT can be integrated into the clinical care of patients with HF in accordance with major society guidelines, as well as future uses of this modality.
Discussion
The core use of cardiac CT to date is in ruling out CAD. Coronary artery calcium (CAC) scans, which are rapid, safe, and non-contrast CT scans that can be used in predicting cardiovascular risk. The 2018 ACC/AHA Cholesterol Guideline states that coronary artery calcium (CAC) testing may be considered in adults with intermediate risk [2]. Regarding patients with HF, a prospective, blinded study showed that CAC scans have sensitivity of 99% and overall accuracy of 92% in patients with LVEF <40% [3]. This is one way to rule out an ischemic etiology of HF in patients with low-intermediate risk of CAD.
While CAC scans are useful, computed coronary tomographic angiography (CCTA) is even more robust. CCTA is another form of CT scan that?s fast (occurring in a single breath hold), extremely accurate, and safe (Figures 1-3). One large multi-centered prospective trial showed that CCTA has sensitivity, specificity, negative predictive value, and positive predictive value of 92-100%, 83-93%, 88-100%, 81-92%, respectively, for detection of coronary lesions with >50% luminal stenosis [4]. With regard to safety, the radiation does is relatively low at 3.5msV and CCTA has been proven to be safer than invasive coronary angiography [4,5]. Lastly, CCTA is widely applicable and retains accuracy (albeit with decreased sensitivity) in patients with atrial fibrillation, acute decompensated heart failure, prior history of CAD with stents, and end stage renal disease [6-9].
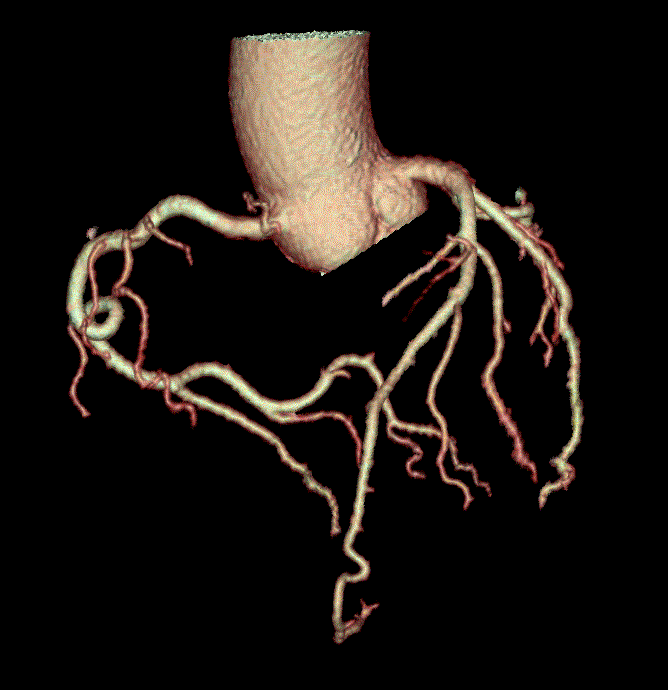
Figure 1: Three-dimensional rendering of CCTA.
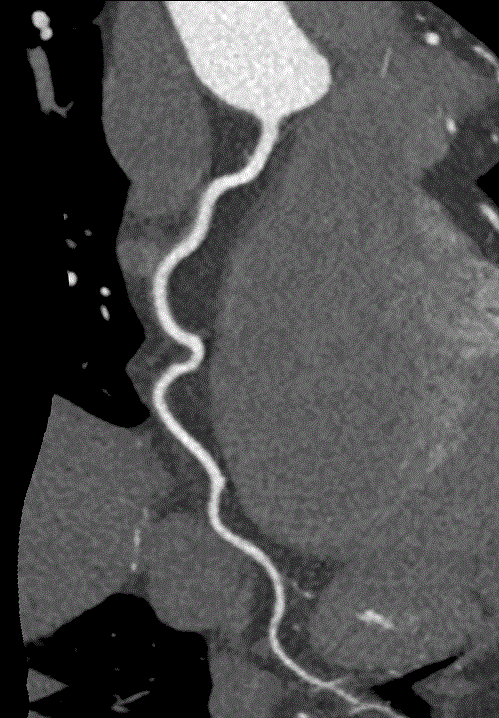
Figure 2: CCTA image of right coronary artery.
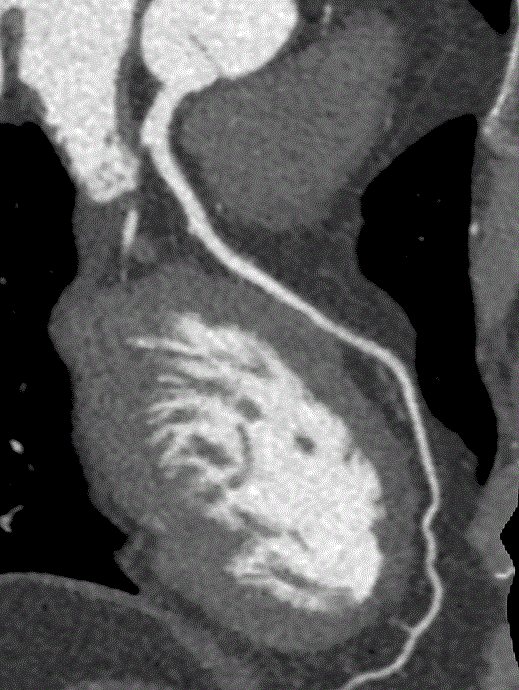
Figure 3: CCTA image of left anterior descending artery.
The 2021 AHA/ACC guidelines on chest pain list CCTA is a class 1A recommendation for patients with acute chest pain and no known CAD, after a negative or inconclusive workup for acute coronary syndrome. It?s also considered a key element in the initial workup of heart failure by the ESC guidelines [10,11]. Although both along with nuclear myocardial perfusion imaging (nMPI) and CCTA have their respective strengths, it should be noted that in the evidence to date, CCTA has outperformed nMPI in terms of accuracy for detecting CAD [12], and CCTA is a Class 1a, while nMPI has been reduced to a Class Ib recommendation.
A second myocardial technique called perfusion scanning can also be used to evaluate CAD that effects blood flow to the myocardium (Figure 4) [13]. One paper considered whether cardiac CT might be considered a ?one stop shop? for CAD imaging [14]. This model suggests that combined protocols be used to identify coronary artery luminal stenosis, deficits in myocardial perfusion, and myocardial scar at the same time. Currently, there may be limitations to this approach related to high contrast load and radiation exposure. 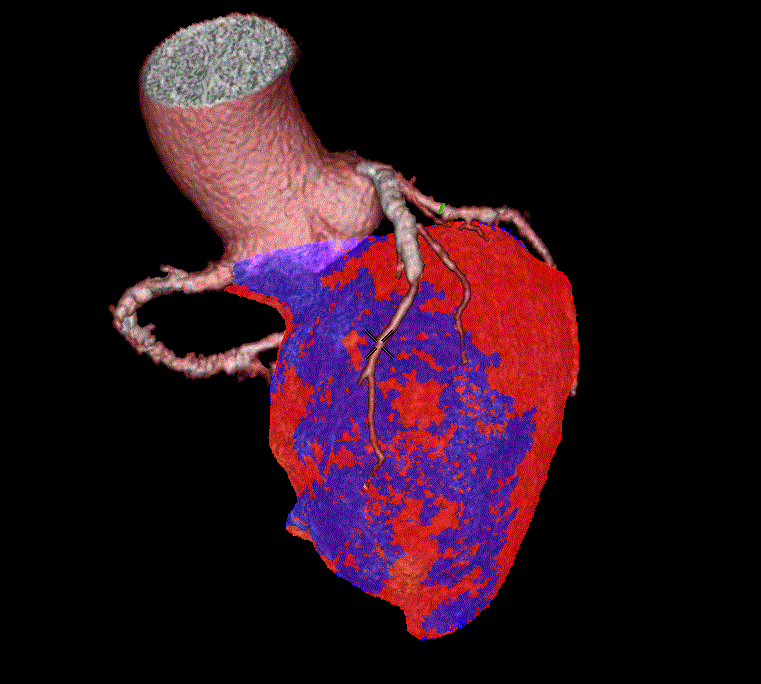
Figure 4: Three-dimensional rendering of myocardial CT perfusion scanning.
Another use of cardiac CT has been to image myocardial fibrosis using a technique called myocardial CT delayed enhancement (CTDE). One example is the imaging of myocardial scar related to ischemic heart disease. Magnetic resonance imaging (MRI) with late gadolinium enhancement (LGE) has been the gold standard in this field, and myocardial CTDE has comparable performance to cardiac MRI [15]. Because fibrosis represents the end stage manifestation of multiple disease pathways (such as aortic stenosis, cardiac sarcoidosis, cardiac amyloidosis, etc), myocardial CTDE has a variety of possible uses, many of which are still evolving and are not yet included in major society guidelines. In general, MRI supersedes myocardial CTDE in this arena, and so myocardial CTDE can be thought of as an alternative to MRI where cost, access, or expertise prevent MRI acquisition.
Next, cardiac CT has numerous applications in structural and electrical cardiac disease. The basis for these applications is the excellent spatial and temporal resolution of cardiac CT. It is considered vital to any TAVR program, as it is the gold standard for aortic annular sizing (Figure 5) [16]. Moreover, cardiac CT can be used peri-procedurally in transcatheter mitral valve replacement device selection, left atrial appendage occlusion planning, and cardiac resynchronization therapy lead placement [17-19].
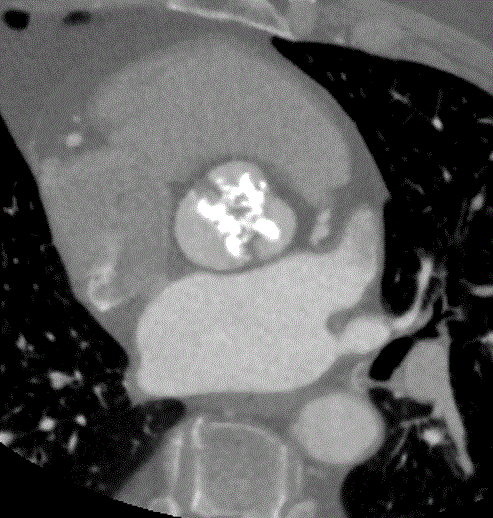
Figure 5: Cardiac CT image of calcified aortic valve.
Future uses of cardiac CT include hardware improvements to for more detailed anatomic images, improved perfusion imaging, and upgraded assessment of myocardial viability. It is also possible that it will be more routinely used peri-procedurally. Specifically, cardiac CT is a rapid imaging modality to assist radiofrequency catheter ablation planning in lieu of MRI in cases where patients do not have MRI compatible devices or are too sick to spend much time in the MRI scanner [20]. Another example was recently published, whereby three-dimensional images from CT can be synchronized with two dimensional fluoroscopic images in the catheterization laboratory to guide interventionalists intra-procedurally [21].
Current limitations of cardiac CT include radiation exposure, which is decreasing with time. Furthermore, the use of iodine contrast in certain protocols can make providers hesitant to utilize this modality in patients with kidney disease. Lastly, more work needs to be done on standardizing cut-offs and protocols for the more recent applications of this modality.
Conclusion
In summary, cardiac CT is a viable, robust, and versatile tool to assist in the complicated care of patients with HF.
References
1. Kitchin SS, Manubolu VS, Roy SK, Budoff MJ. The Role of Cardiac Computed Tomography in Heart Failure. Current Heart Failure Reports. 2022 May ;19(4):213–22. https://doi.org/10.1007/s11897-022-00553-2
2. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, De Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Jun 18;139(25):e1082-143. https://doi.org/10.1161/CIR.0000000000000625
3. Budoff MJ, Shavelle DM, Lamont DH, Kim HT, Akinwale P, Kennedy JM, Brundage BH. Usefulness of electron beam computed tomography scanning for distinguishing ischemic from nonischemic cardiomyopathy. Journal of the American College of Cardiology. 1998 Nov 1;32(5):1173-8.
4. Lee D, Li D, Jug B, Papazian J, Budoff M. Diagnostic accuracy of 64 slice multidetector coronary computed tomographic angiography in left ventricular systolic dysfunction. IJC Heart & Vasculature. 2015 Sep 1;8:42-6. https://doi.org/10.1016/j.ijcha.2015.04.007
5. Andreini D, Pontone G, Bartorelli AL, Agostoni P, Mushtaq S, Bertella E, Trabattoni D, Cattadori G, Cortinovis S, Annoni A, Castelli A. Sixty-four–slice multidetector computed tomography: an accurate imaging modality for the evaluation of coronary arteries in dilated cardiomyopathy of unknown etiology. Circulation: Cardiovascular Imaging. 2009 May;2(3):199-205. https://doi.org/10.1161/CIRCIMAGING.108.822809
6. Sun ML, Lu B, Wu RZ, Johnson L, Han L, Liu G, Yu FF, Hou ZH, Gao Y, Wang HY, Jiang S. Diagnostic accuracy of dual-source CT coronary angiography with prospective ECG-triggering on different heart rate patients. European radiology. 2011 Aug;21(8):1635-42. https://doi.org/10.1007/s00330-011-2107-5
7. Oncel D, Oncel G, Tastan A. Effectiveness of dual-source CT coronary angiography for the evaluation of coronary artery disease in patients with atrial fibrillation: initial experience. Radiology. 2007 Dec;245(3):703-11. https://doi.org/10.1148/radiol.2453070094
8. Abdelkarim MJ, Ahmadi N, Gopal A, Hamirani Y, Karlsberg RP, Budoff MJ. Noninvasive quantitative evaluation of coronary artery stent patency using 64-row multidetector computed tomography. Journal of Cardiovascular Computed Tomography. 2010 Jan 1;4(1):29-37. https://doi.org/10.1016/j.jcct.2009.10.014
9. Jug B, Papazian J, Gupta M, Bhatia H, Derakhshani A, Koplik S, Karlsberg RP, Budoff MJ. Diagnostic performance of computed tomographic coronary angiography in patients with end-stage renal disease. Coronary artery disease. 2013 Mar 1;24(2):135-41. https://doi.org/10.1097/MCA.0b013e32835be39a
10. Members WC, Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2021 Nov 30;78(22):2218-61. https://doi.org/10.1016/j.jacc.2021.07.052
11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, ?elutkien? J, Chioncel O, Cleland JG. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European heart journal. 2021 Sep 21;42(36):3599-726. https://doi.org/10.1093/eurheartj/ehab368
12. Budoff MJ, Li D, Kazerooni EA, Thomas GS, Mieres JH, Shaw LJ. Diagnostic accuracy of noninvasive 64-row computed tomographic coronary angiography (CCTA) compared with myocardial perfusion imaging (MPI): the PICTURE study, a prospective multicenter trial. Academic Radiology. 2017 Jan 1;24(1):22-9. https://doi.org/10.1016/j.acra.2016.09.008
13. Li Y, Yu M, Dai X, Lu Z, Shen C, Wang Y, Lu B, Zhang J. Detection of hemodynamically significant coronary stenosis: CT myocardial perfusion versus machine learning CT fractional flow reserve. Radiology. 2019 Nov;293(2):305-14. https://doi.org/10.1148/radiol.2019190098
14. Danad I, Min JK. Computed tomography: The optimal imaging method for differentiation of ischemic vs non-ischemic cardiomyopathy. Journal of Nuclear Cardiology. 2015 Oct;22(5):961-7. https://doi.org/10.1007/s12350-015-0146-z
15. Ohta Y, Kitao S, Yunaga H, Fujii S, Mukai N, Yamamoto K, Ogawa T. Myocardial delayed enhancement CT for the evaluation of heart failure: comparison to MRI. Radiology. 2018 Sep;288(3):682-91. https://doi.org/10.1148/radiol.2018172523
16. Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, Marwan M, Nørgaard BL, Piazza N, Schoenhagen P, Leipsic JA. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR) an expert consensus document of the Society of Cardiovascular Computed Tomography. JACC: Cardiovascular Imaging. 2019 Jan;12(1):1-24. https://doi.org/10.1016/j.jcmg.2018.12.003
17. Shanks M, Delgado V, Ng AC, Van Der Kley F, Schuijf JD, Boersma E, Van De Veire NR, Nucifora G, Bertini M, De Roos A, Kroft L. Mitral valve morphology assessment: three-dimensional transesophageal echocardiography versus computed tomography. The Annals of thoracic surgery. 2010 Dec 1;90(6):1922-9. https://doi.org/10.1016/j.athoracsur.2010.06.116
18. Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circulation: Cardiovascular Imaging. 2013 Mar;6(2):185-94. https://doi.org/10.1161/CIRCIMAGING.112.000153
19. Nguyen UC, Cluitmans MJ, Strik M, Luermans JG, Gommers S, Wildberger JE, Bekkers SC, Volders PG, Mihl C, Prinzen FW, Vernooy K. Integration of cardiac magnetic resonance imaging, electrocardiographic imaging, and coronary venous computed tomography angiography for guidance of left ventricular lead positioning. EP Europace. 2019 Apr 1;21(4):626-35. https://doi.org/10.1093/europace/euy292
20. Conte E, Mushtaq S, Carbucicchio C, Piperno G, Catto V, Mancini ME, Formenti A, Annoni A, Guglielmo M, Baggiano A, Muscogiuri G. State of the art paper: Cardiovascular CT for planning ventricular tachycardia ablation procedures. Journal of Cardiovascular Computed Tomography. 2021 Sep 1;15(5):394-402. https://doi.org/10.1016/j.jcct.2021.01.002
21. Collet C, Sonck J, Leipsic J, Monizzi G, Buytaert D, Kitslaar P, Andreini D, De Bruyne B. Implementing coronary computed tomography angiography in the catheterization laboratory. Cardiovascular Imaging. 2021 Sep 1;14(9):1846-55. https://doi.org/10.1016/j.jcmg.2020.07.048
