Genetic Mechanisms of Neurodevelopmental Risk in Congenital Heart Disease: Mostly Unknowns
Authors: Sarah E. Hanna1, Tina O. Findley2#, Jacqueline G. Parchem3#, Sarah U. Morton1,4*#
1Division of Newborn Medicine, Boston Children’s Hospital, Boston, Massachusetts, USA
2Division of Neonatal-Perinatal Medicine, Department of Pediatrics, McGovern Medical School at The University of Texas Health Science Center at Houston and Children’s Memorial Hermann Hospital, Houston, Texas, USA
3Division of Maternal-Fetal Medicine, Department of Obstetrics, Gynecology, and Reproductive Sciences, McGovern Medical School at The University of Texas Health Science Center at Houston, USA
4Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA
#These authors contributed equally
*Corresponding author: Sarah U Morton, Boston Children’s Hospital, 300 Longwood Ave, Boston MA 02115; USA; E-mail: Sarah.Morton@childrens.harvard.edu
Received: 21 August 2023; Revised: 30 August 2023; Accepted: 31 August 2023; Published: 31 August 2023
Copyright: © 2023 Hanna SE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Running Title: Genetics of Neurodevelopmental in Congenital Heart Disease
Citation: Hanna SE, Findley TO, Parchem JG, Morton SU. Genetic Mechanisms of Neurodevelopmental Risk in Congenital Heart Disease: Mostly Unknowns. 21st Century Cardiol. 2023 August; 3(4):142
Abstract
Congenital heart disease (CHD) is the most common major congenital anomaly, occurring in approximately one percent of live births globally1,2. Yet, an underlying genetic etiology is identified in only 20 to 30 percent of people with CHD3,4. The role of genetic factors in CHD risk is demonstrated by the high recurrence rate within families and its association with genetic syndromes5,6. CHD is associated with a higher incidence of neurodevelopmental delay and disability (NDD)7–11, and there has been recent interest in the contribution of genetics to these outcomes12,13. As the survival rate for individuals with CHD has significantly improved14, the excess risk for impairments in motor skills, language abilities, and social-emotional functioning have been become more apparent11,15–19. Both factors intrinsic to an individual patient, such as genetic variants or structural type of CHD, as well as extrinsic factors, such as nutritional supports and anesthesia exposures, can influence the risk of NDD20,21. However, as few multicenter trials include patients with known genetic diagnoses or genomic data22, we have a limited understanding of how genetic variation contributes to neurodevelopmental outcomes among individuals with CHD.
Keywords:
Congenital heart disease; Genetics; Neurodevelopment
Introduction
Congenital heart disease (CHD) is the most common major congenital anomaly, occurring in approximately one percent of live births globally [1,2]. Yet, an underlying genetic etiology is identified in only 20 to 30 percent of people with CHD [3,4]. The role of genetic factors in CHD risk is demonstrated by the high recurrence rate within families and its association with genetic syndromes [5,6]. CHD is associated with a higher incidence of neurodevelopmental delay and disability (NDD) [7-11], and there has been recent interest in the contribution of genetics to these outcomes [12,13]. As the survival rate for individuals with CHD has significantly improved [14], the excess risk for impairments in motor skills, language abilities, and social-emotional functioning have been become more apparent [11,15-19]. Both factors intrinsic to an individual patient, such as genetic variants or structural type of CHD, as well as extrinsic factors, such as nutritional supports and anesthesia exposures, can influence the risk of NDD [20,21]. However, as few multicenter trials include patients with known genetic diagnoses or genomic data [22], we have a limited understanding of how genetic variation contributes to neurodevelopmental outcomes among individuals with CHD.
Early Alterations in Neurodevelopment
Recent studies suggest that the effects of CHD on brain development begin in utero [23-25]. Fetal brain volume as measured by magnetic resonance imaging is decreased in CHD, and in the overall cohort those with lower brain volume had lower scores for cognitive, language, motor, and adaptive functioning at two years of age [26,27]. In a multivariable model adjusting for socioeconomic status and postnatal factors such as surgical bypass time and length of initial hospitalization, fetal brain volume remained the most consistent predictor of neurodevelopmental outcomes, explaining 18% to 45% of the variance in outcomes. Similarly, studies utilizing pre-operative MRIs of infants with CHD have demonstrated evidence of early ischemic injury prior to CHD surgery [11,23,28]. These findings underscore the importance of considering prenatal factors in the assessment of neurodevelopmental risk.
The impact of certain risk factors for adverse neurodevelopmental outcomes has also been investigated in CHD patients, including timing of major surgery and anesthesia exposure [29-31], and fetal or postnatal hypoxic injury in cyanotic phenotypes [26,27,32-34]. There are multiple mechanisms by which CHD affects brain development, including intrinsic factors, e.g. genetics and CHD type [12,13], as well as extrinsic factors such as surgical/medical interventions, inflammatory responses, and environment exposures [15,20,23] (Figure 1).
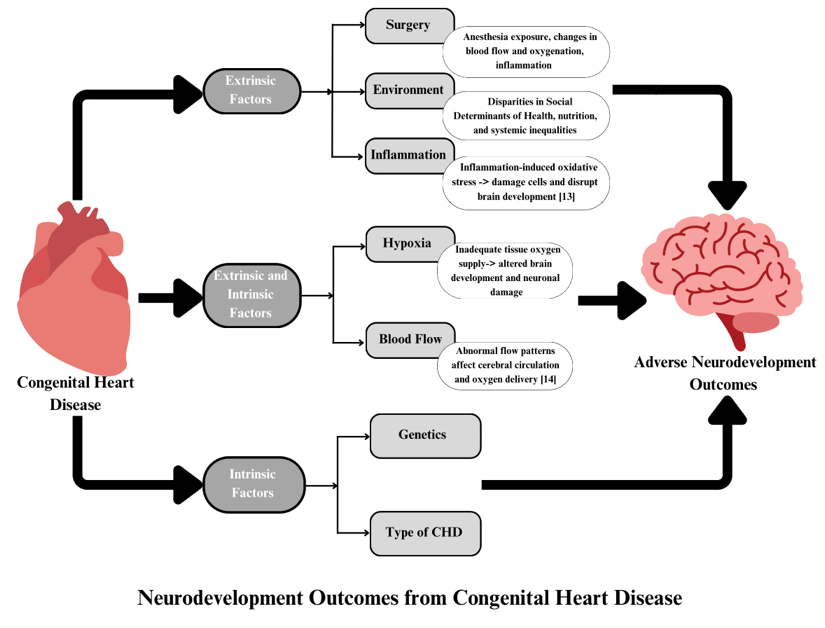
Figure 1: Summary of factors that could influence neurodevelopment among persons with congenital heart disease. Intrinsic factors are those which are not currently easy to modify, such as patient genetic variants or the structural type of CHD. However, the downstream mechanisms by which these factors impact NDD may be targets for therapeutic interventions. By contrast, many extrinsic factors could be targeted directly to mitigate NDD risk.
Additionally, factors such as hypoxia and altered blood flow [20,35,36], which can be considered both intrinsic and extrinsic to CHD, have been identified as potentially modifiable factors that may impact brain development in CHD individuals. As genetic causes of CHD could also directly cause NDD via influences on in utero brain development, or by impacting neuronal resiliency during exposure to stressors such as hypoxia or surgery, it remains unclear to what extent the variability in outcomes due to early life exposure are attributable to intrinsic genetic risk.
Genetic Risk and Neurodevelopment
The relationship between CHD, genetics, and neurodevelopment is complex. Roughly 440 genes are known to cause human CHD, including genes encoding transcription factors (GATA4, NKX2-5, TBX5), cell signaling molecules (NOTCH1), structural proteins (VEGF), chromatin modifiers (KMT2D), and cilia-related proteins (NODAL) [4,37-40]. Genetic variants that cause CHD may also directly influence brain development [3,11,13,41]. For example, specific genetic variants such as copy number variants involving 1q21.1, 16p12.1-11, and 8p23.1 have been identified in people who have high rates of both CHD and neurodevelopmental disorders. Due to limited integration of genetic risk in clinical studies of CHD outcomes, the exact mechanisms by which genetic changes alter human development are often not known [39,42,43].
Two common genetic causes of CHD, Trisomy 21 [44-47] (T21, also known as Down syndrome) and 22q11 deletion syndrome [48-51] (22q11DS, also known as DiGeorge or velocardiofacial syndrome), are associated with increased risk of both CHD and neurodevelopmental delay or disability. As both disorders result in the dysregulation of many genes, it could be that either single genes or multiple genes lead to the shared risk to heart and brain development. Human chromosome 21 contains more than 500 genes, including DSCAM (Down Syndrome Cell Adhesion Molecule) and COL6A (Collagen VI). Overexpression of DSCAM and COL6A leads to CHD in mice [3,52], while copy number variants involving DSCAM have been associated with intellectual disability, autism, and bipolar disorder [53]. Similarly, the TBX1 gene within the 22q11DS locus is associated with CHD [54]. Findings from mouse models have also revealed that loss of TBX1 results in deficits in social interaction and communication, impaired working memory, and heightened anxiety [55,56]. In one study, people with 22q11DS had a longer interoperative bypass time, highlighting the potential interaction between intrinsic genetic variants and extrinsic medical factors [57.] Further research into the multifaceted impact of genes relevant to T21 and 22q11DS on both cardiac and neurological development holds promise for developing targeted interventions for individuals with CHD.
Once single-gene disorder that often includes CHD and neurodevelopmental disorders is CHARGE (ocular coloboma, heart malformations, atresia of the choanae, retardation of growth, genital hypoplasia, and ear abnormalities). CHARGE is an autosomal dominant disorder resulting from variants in CHD7, a chromatin helicase [58-60]. Developmentally, CHD7 is expressed in the neural crest, and this broad expression is hypothesized to account for the observed pleiotropy [61,62]. However, there is limited understanding of genotype-phenotype associations to explain the variation in neurological and psychiatric outcomes for individuals with CHARGE who also have CHD.
Conclusion
In conclusion, even among established genetic causes of CHD, we have a limited understanding of the molecular and cellular mechanisms underlying the shared risk to heart and neurodevelopment. Beyond genetics, the role of extrinsic factors, such as the in-utero environment [23] and social determinants of health [63] also contribute to the complexity of neurodevelopmental outcomes among people with CHD. Modifying NDD risk factors, such adjunctive therapies during surgery that maximize neuroprotection [64] or pharmacogenetically-informed medication choices that reduce off-target effects [65], provide opportunities for novel interventions to reduce NDD. As many studies exclude participants with known genetic diagnoses, we have a limited understanding of the relationship which NDD risk factors may be relevant to patients with genetic disorders such as T21 or 22q11DS, nor the molecular mechanisms by which any risk factor is contributing to NDD. Designing studies to include prenatal, genetic, and postnatal factors are needed to build a more comprehensive model of the interplay between these factors and ensure that results can be translated to meaningful clinical interventions. Future research should aim to comprehensively consider the interplay of genetics, extrinsic, and intrinsic factors.
References
1. Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr. 2008 Dec 1;153(6):807-13. https://doi.org/10.1016/j.jpeds.2008.05.059
2. Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014 Aug 26;130(9):749-56. https://doi.org/10.1161/CIRCULATIONAHA.113.008396
3. Zaidi S, Brueckner M. Genetics and genomics of congenital heart disease. Circ Res. 2017 Mar 17;120(6):923-40. https://doi.org/10.1161/CIRCRESAHA.116.309140
4. Jin SC, Homsy J, Zaidi S, Lu Q, Morton S, DePalma SR, et al. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet. 2017 Nov 1;49(11):1593-601. https://doi.org/10.1038/ng.3970
5. Øyen N, Poulsen G, Boyd HA, Wohlfahrt J, Jensen PK, Melbye M. Recurrence of congenital heart defects in families. Circulation. 2009 Jul 28;120(4):295-301. https://doi.org/10.1161/CIRCULATIONAHA.109.857987
6. Brodwall K, Greve G, Leirgul E, Tell GS, Vollset SE, Øyen N. Recurrence of congenital heart defects among siblings—a nationwide study. Am J Med Genet Part A. 2017 Jun;173(6):1575-85. https://doi.org/10.1002/ajmg.a.38237
7. Calderon J, Newburger JW, Rollins CK. Neurodevelopmental and mental health outcomes in patients with fontan circulation: a state-of-the-art review. Front Pediatr. 2022 Mar 9;10:826349. https://doi.org/10.3389/fped.2022.826349
8. Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015 May 1;135(5):816-25. https://doi.org/10.1542/peds.2014-3825
9. Verrall CE, Blue GM, Loughran-Fowlds A, Kasparian N, Gecz J, Walker K, et al. ‘Big issues’ in neurodevelopment for children and adults with congenital heart disease. Open Heart. 2019 Jul 1;6(2):e000998. https://doi.org/10.1136/openhrt-2018-000998
10. Asschenfeldt B, Evald L, Heiberg J, Salvig C, Østergaard L, Dalby RB, et al. Neuropsychological status and structural brain imaging in adults with simple congenital heart defects closed in childhood. J Am Heart Assoc. 2020 Jun 2;9(11):e015843. https://doi.org/10.1161/JAHA.120.015843
11. Morton PD, Ishibashi N, Jonas RA. Neurodevelopmental abnormalities and congenital heart disease: insights into altered brain maturation. Circ Res. 2017 Mar 17;120(6):960-77. https://doi.org/10.1161/CIRCRESAHA.116.309048
12. Blue GM, Ip E, Walker K, Kirk EP, Loughran-Fowlds A, Sholler GF, et al. Genetic burden and associations with adverse neurodevelopment in neonates with congenital heart disease. Am Heart J. 2018 Jul 1;201:33-9. https://doi.org/10.1016/j.ahj.2018.03.021
13. Nattel SN, Adrianzen L, Kessler EC, Andelfinger G, Dehaes M, Côté-Corriveau G, et al. Congenital heart disease and neurodevelopment: clinical manifestations, genetics, mechanisms, and implications. Can J Cardiol. 2017 Dec 1;33(12):1543-55. https://doi.org/10.1016/j.cjca.2017.09.020
14. Dellborg M, Giang KW, Eriksson P, Liden H, Fedchenko M, Ahnfelt A, et al. Adults with congenital heart disease: trends in event-free survival past middle age. Circulation. 2023 Mar 21;147(12):930-8. https://doi.org/10.1161/CIRCULATIONAHA.122.060834
15. Hövels-Gürich HH. Factors influencing neurodevelopment after cardiac surgery during infancy. Front Pediatr. 2016 Dec 15;4:137. https://doi.org/10.3389/fped.2016.00137
16. Schmithorst VJ, Panigrahy A, Gaynor JW, Watson CG, Lee V, Bellinger DC, et al. Organizational topology of brain and its relationship to ADHD in adolescents with d?transposition of the great arteries. Brain and Behav. 2016 Aug;6(8):e00504. https://doi.org/10.1002/brb3.504
17. Panigrahy A, Schmithorst VJ, Wisnowski JL, Watson CG, Bellinger DC, Newburger JW, et al. Relationship of white matter network topology and cognitive outcome in adolescents with d-transposition of the great arteries. NeuroImage: Clin. 2015 Jan 1;7:438-48. https://doi.org/10.1016/j.nicl.2015.01.013
18. Rollins CK, Asaro LA, Akhondi-Asl A, Kussman BD, Rivkin MJ, Bellinger DC, et al. White matter volume predicts language development in congenital heart disease. J Pediatr. 2017 Feb 1;181:42-8. https://doi.org/10.1016/j.jpeds.2016.09.070
19. Peyvandi S, Latal B, Miller SP, McQuillen PS. The neonatal brain in critical congenital heart disease: insights and future directions. Neuroimage. 2019 Jan 15;185:776-82. https://doi.org/10.1016/j.neuroimage.2018.05.045
20. Selvanathan T, Smith JM, Miller SP, Field TS. Neurodevelopment and cognition across the lifespan in patients with single-ventricle physiology: abnormal brain maturation and accumulation of brain injuries. Can J Cardiol. 2022 Jul 1;38(7):977-87. https://doi.org/10.1016/j.cjca.2022.02.009
21. Peyvandi S, Kim H, Lau J, Barkovich AJ, Campbell A, Miller S, et al. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J Thorac Cardiovasc Surg. 2018 Jan 1;155(1):291-300. https://doi.org/10.1016/j.jtcvs.2017.08.019
22. Morton SU, Norris-Brilliant A, Cunningham S, King E, Goldmuntz E, Brueckner M, et al. Association of Potentially Damaging De Novo Gene Variants with Neurologic Outcomes in Congenital Heart Disease. JAMA Netw Open. 2023 Jan 3;6(1):e2253191. https://doi.org/10.1001/jamanetworkopen.2022.53191
23. Leon RL, Mir IN, Herrera CL, Sharma K, Spong CY, Twickler DM, et al. Neuroplacentology in congenital heart disease: placental connections to neurodevelopmental outcomes. Pediatr Res. 2022 Mar;91(4):787-94. https://doi.org/10.1038/s41390-021-01521-7
24. Van Nisselrooij AE, Jansen FA, van Geloven N, Linskens IH, Pajkrt E, Clur SA, et al. Impact of extracardiac pathology on head growth in fetuses with congenital heart defect. Ultrasound in Obstet Gynecol. 2020 Feb;55(2):217-25. https://doi.org/10.1002/uog.20260
25. Hansen T, Henriksen TB, Bach CC, Matthiesen NB. Congenital heart defects and measures of prenatal brain growth: a systematic review. Pediatr Neurol. 2017 Jul 1;72:7-18. https://doi.org/10.1016/j.pediatrneurol.2017.03.014
26. Sadhwani A, Wypij D, Rofeberg V, Gholipour A, Mittleman M, Rohde J, et al. Fetal brain volume predicts neurodevelopment in congenital heart disease. Circulation. 2022 Apr 12;145(15):1108-19. https://doi.org/10.1161/CIRCULATIONAHA.121.056305
27. Peyvandi S, Rollins C. Fetal brain development in congenital heart disease. Can J Cardiol. 2023 Feb 1;39(2):115-22. https://doi.org/10.1016/j.cjca.2022.09.020
28. Beca J, Gunn J, Coleman L, Hope A, Whelan LC, Gentles T, et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol. 2009 May 12;53(19):1807-11. https://doi.org/10.1016/j.jacc.2009.01.061
29. Huisenga D, La Bastide?Van Gemert S, Van Bergen A, Sweeney J, Hadders?Algra M. Developmental outcomes after early surgery for complex congenital heart disease: A systematic review and meta?analysis. Dev Med Child Neurol. 2021 Jan;63(1):29-46. https://doi.org/10.1111/dmcn.14512
30. Gunn JK, Beca J, Hunt RW, Goldsworthy M, Brizard CP, Finucane K, et al. Perioperative risk factors for impaired neurodevelopment after cardiac surgery in early infancy. Arch Dis Child. 2016 Nov 1;101(11):1010-6. https://doi.org/10.1136/archdischild-2015-309449
31. Lisanti AJ, Vittner DJ, Peterson J, Van Bergen AH, Miller TA, Gordon EE, et al. Developmental care pathway for hospitalised infants with CHD: on behalf of the Cardiac Newborn Neuroprotective Network, a Special Interest Group of the Cardiac Neurodevelopmental Outcome Collaborative. Cardiol Young. 2023 Mar 30:1-18. https://doi.org/10.1017/s1047951123000525
32. Ramirez A, Peyvandi S, Cox S, Gano D, Xu D, Tymofiyeva O, et al. Neonatal brain injury influences structural connectivity and childhood functional outcomes. PLoS One. 2022 Jan 5;17(1):e0262310. https://doi.org/10.1371/journal.pone.0262310
33. Seed M, Limperopoulos C. In Utero Brain Growth Matters for Fetuses with Congenital Heart Disease. Circulation. 2022 Apr 12;145(15):1120-2. https://doi.org/10.1161/CIRCULATIONAHA.122.058683
34. Bonthrone AF, Kelly CJ, Ng IH, Counsell SJ. MRI studies of brain size and growth in individuals with congenital heart disease. Trans Pediatr. 2021 Aug;10(8):2171-2181. https://doi.org/10.21037/tp-20-282
35. Cheng HH, Wypij D, Laussen PC, Bellinger DC, Stopp CD, Soul JS, et al. Cerebral blood flow velocity and neurodevelopmental outcome in infants undergoing surgery for congenital heart disease. Ann Thorac Surg. 2014 Jul 1;98(1):125-32. https://doi.org/10.1016/j.athoracsur.2014.03.035
36. Sadhwani A, Cheng H, Stopp C, Rollins CK, Jolley MA, Dunbar-Masterson C, et al. Early neurodevelopmental outcomes in children supported with ECMO for cardiac indications. Pediatr Cardiol. 2019 Jun 15;40:1072-83. https://doi.org/10.1007/s00246-019-02115-1
37. Homsy J, Zaidi S, Shen Y, Ware JS, Samocha KE, Karczewski KJ, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. 2015 Dec 4;350(6265):1262-6. https://doi.org/10.1126/science.aac9396
38. Watkins WS, Hernandez EJ, Wesolowski S, Bisgrove BW, Sunderland RT, Lin E, et al. De novo and recessive forms of congenital heart disease have distinct genetic and phenotypic landscapes. Nat Commun. 2019 Oct 17;10(1):4722. https://doi.org/10.1038/s41467-019-12582-y
39. Diab NS, Barish S, Dong W, Zhao S, Allington G, Yu X, et al. Molecular genetics and complex inheritance of congenital heart disease. Genes. 2021 Jun 30;12(7):1020. https://doi.org/10.3390/genes12071020
40. Morton SU, Quiat D, Seidman JG, Seidman CE. Genomic frontiers in congenital heart disease. Nat Rev Cardiol. 2022 Jan;19(1):26-42. https://doi.org/10.1038/s41569-021-00587-4
41. Spielmann N, Miller G, Oprea TI, Hsu CW, Fobo G, Frishman G, et al. Extensive identification of genes involved in congenital and structural heart disorders and cardiomyopathy. Nat Cardiovasc Res. 2022 Feb;1(2):157-73. https://doi.org/10.1038/s44161-022-00018-8
42. Nees SN, Chung WK. The genetics of isolated congenital heart disease. Am J Med Gen Part C: Sem Med Genet. 2020 Mar;184(1): 97-106. https://doi.org/10.1002/ajmg.c.31763
43. Choudhury TZ, Garg V. Molecular genetic mechanisms of congenital heart disease. Curr Opin Genet Dev. 2022 Aug 1;75:101949. https://doi.org/10.1016/j.gde.2022.101949
44. Dimopoulos K, Constantine A, Clift P, Condliffe R, Moledina S, Jansen K, et al. Cardiovascular complications of down syndrome: scoping review and expert consensus. Circulation. 2023 Jan 31;147(5):425-41. https://doi.org/10.1161/CIRCULATIONAHA.122.059706
45. Delany DR, Gaydos SS, Romeo DA, Henderson HT, Fogg KL, McKeta Aset al. Down syndrome and congenital heart disease: perioperative planning and management. J Congenit Cardiol. 2021 Dec;5:1-4. https://doi.org/10.1186/s40949-021-00061-3
46. Patt E, Singhania A, Roberts AE, Morton SU. The genetics of neurodevelopment in congenital heart disease. Can J Cardiol. 2023 Feb 1;39(2):97-114. https://doi.org/10.1016/j.cjca.2022.09.026
47. Kovacs AH, Brouillette J, Ibeziako P, Jackson JL, Kasparian NA, Kim YY, et al. Psychological outcomes and interventions for individuals with congenital heart disease: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2022 Aug;15(8):672-688. https://doi.org/10.1161/HCQ.0000000000000110
48. Goldmuntz E. 22q11. 2 deletion syndrome and congenital heart disease. Am J Med Gent Part C: Seminars in Medical Genetics 2020 Mar;184(1):64-72). Hoboken, USA: John Wiley & Sons, Inc.. https://doi.org/10.1002/ajmg.c.31774
49. Unolt M, Versacci P, Anaclerio S, Lambiase C, Calcagni G, Trezzi M, et al. Congenital heart diseases and cardiovascular abnormalities in 22q11. 2 deletion syndrome: from well?established knowledge to new frontiers. Am J Med Genet Part A. 2018 Oct;176(10):2087-98. https://doi.org/10.1002/ajmg.a.38662
50. Davies RW, Fiksinski AM, Breetvelt EJ, Williams NM, Hooper SR, Monfeuga T, et al. Using common genetic variation to examine phenotypic expression and risk prediction in 22q11. 2 deletion syndrome. Nat Med. 2020 Dec;26(12):1912-8. https://doi.org/10.1038/s41591-020-1103-1
51. Cheung EN, George SR, Andrade DM, Chow EW, Silversides CK, Bassett AS. Neonatal hypocalcemia, neonatal seizures, and intellectual disability in 22q11. 2 deletion syndrome. Genet Med. 2014 Jan;16(1):40-4. https://doi.org/10.1038/gim.2013.71
52. Mollo N, Scognamiglio R, Conti A, Paladino S, Nitsch L, Izzo A. Genetics and molecular basis of congenital heart defects in down syndrome: Role of extracellular matrix regulation. Int J Mol Sci. 2023 Feb 2;24(3):2918. https://doi.org/10.3390/ijms24032918
53. Mitsogiannis MD, Pancho A, Aerts T, Sachse SM, Vanlaer R, Noterdaeme L, et al. Subtle roles of down syndrome cell adhesion molecules in embryonic forebrain development and neuronal migration. Front Cell Dev Biol. 2021 Jan 28;8:624181. https://doi.org/10.3389/fcell.2020.624181
54. Xu H, Morishima M, Wylie JN, Schwartz RJ, Bruneau BG, Lindsay EA, et al. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131(13):3217-27. https://doi.org/10.1242/dev.01174
55. Zinkstok JR, Boot E, Bassett AS, Hiroi N, Butcher NJ, Vingerhoets C, et al. Neurobiological perspective of 22q11. 2 deletion syndrome. Lancet Psychiatry. 2019 Nov 1;6(11):951-60. https://doi.org/10.1016/S2215-0366(19)30076-8
56. Zhang M, Li FX, Liu XY, Hou JY, Ni SH, Wang J, et al. TBX1 loss?of?function mutation contributes to congenital conotruncal defects. Exp Ther Med. 2018 Jan 1;15(1):447-53. https://doi.org/10.3892/etm.2017.5362
57. Mercer-Rosa L, Pinto N, Yang W, Tanel R, Goldmuntz E. 22q11. 2 Deletion syndrome is associated with perioperative outcome in tetralogy of Fallot. J Thorac Cardiovasc Surg. 2013 Oct 1;146(4):868-73. https://doi.org/10.1016/j.jtcvs.2012.12.028
58. Thomas AT, Waite J, Williams CA, Kirk J, Oliver C, Richards C. Phenotypic characteristics and variability in CHARGE syndrome: a PRISMA compliant systematic review and meta-analysis. J Neurodev Disord 2022 Dec;14(1):49. https://doi.org/10.1186/s11689-022-09459-5
59. Meisner JK, Martin DM. Congenital heart defects in CHARGE: The molecular role of CHD7 and effects on cardiac phenotype and clinical outcomes. Am J Med Genet Part C Semin Med Genet. 2020;184: 81-89. https://doi.org/10.1002/ajmg.c.31761
60. Zentner GE, Layman WS, Martin DM, Scacheri PC. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am J Med Genet Part A. 2010 Mar;152(3):674-86. https://doi.org/10.1002/ajmg.a.33323
61. Martin DM, Sheldon S, Gorski JL. CHARGE association with choanal atresia and inner ear hypoplasia in a child with a de novo chromosome translocation t (2; 7)(p14; q21. 11). Am J Med Genet. 2001 Mar 1;99(2):115-9. https://doi.org/10.1002/1096-8628(2000)9999:999<00::aid-ajmg1126>3.0.co;2-8
62. Layman WS, Hurd EA, Martin DM. Chromodomain proteins in development: lessons from CHARGE syndrome. Clin Genet. 2010 Jul;78(1):11-20. https://doi.org/10.1111/j.1399-0004.2010.01446.x
63. Davey B, Sinha R, Lee JH, Gauthier M, Flores G. Social determinants of health and outcomes for children and adults with congenital heart disease: a systematic review. Pediatr Res. 2021 Jan;89(2):275-94. https://doi.org/10.1038/s41390-020-01196-6
64. Albers EL, Bichell DP, McLaughlin B. New approaches to neuroprotection in infant heart surgery. Pediatr Res. 2010 Jul;68(1):1-9. https://doi.org/10.1203/PDR.0b013e3181df5402
65. de Denus S, Kantor PF. Pharmacogenomics and heart failure in congenital heart disease. Can J Cardiol. 2013 Jul 1;29(7):779-85. https://doi.org/10.1016/j.cjca.2013.04.017