Chronic Kidney Disease and its Impact on Cardiovascular Disease and Treatment Modalities
Authors: Li Zhao1, Ana Maria Waaga-Gasser1,2, Li-Li Hsiao1,2, Amrendra K. Ajay1,2*
1Division of Renal Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA 02115
2Department of Medicine, Harvard Medical School, Boston, MA 02115
*Correspondence to: Amrendra K. Ajay, Department of Medicine, Harvard Medical School, 221 Longwood Ave, BLI447, Boston, MA 02115, USA; E-mail: akajay@bwh.harvard.edu
Received: 07 January 2022; Accepted: 27 February 2022; Published: 28 February 2022
Copyright: © 2022 Zhao Li, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Zhao Li, Waaga-Gasser AM, Hsiao LL and Ajay AK (2022) Chronic Kidney Disease and its impact on cardiovascular disease and treatment modalities. 21st Century Cardiol, Volume 2 (1): 116
Abstract
Kidney dysfunction is one of the most important comorbidities in heart failure. The reduced estimated glomerular filtration rate is a strong predictor of cardiovascular mortality and its associated complications. Chronic Kidney Disease (CKD) is characterized by progressive loss of kidney function, leading to either dialysis or Renal Replacement Therapy (RRT), and is associated with high mortality. The quality of life for patients with dialysis or on RRT is very poor. The primary causes of CKD are diabetes and hypertension. On the other hand, studies show that kidney diseases over time promote profound cardiovascular changes resulting in cardiovascular disease (CVD). The factors that are released from the kidney to cause cardiac phenotype changes are not well understood. The secretory proteins that play a critical role in CKD and how they mediate the progression of CVDs need further attention. In this article we review recent findings of CKD-mediated cardiovascular changes culminating in CVD and its treatment modalities.
Keywords:
Chronic kidney disease; Cardiovascular disease; Cardiorenal syndrome
Chronic kidney disease and risk of cardiovascular disease
Kidney and heart function are closely interconnected physiologically and patho-physiologically, both in healthy and in the diseased state. Kidney function, as reflected by serum creatinine and more precisely the Albumin to Creatinine Ratio (SCR) and the estimated glomerular filtration rate (eGFR), is persistently one of the best predictors of cardiovascular outcomes in many populations [1-3]. This includes high-risk patients with congestive heart failure, post-myocardial infarctions, and those with diabetes mellitus.
In a clinical trial including patients with post-myocardial infarction receiving state of the art therapy with median lower eGFR had a 50% increased risk of cardiovascular events such as death compared with those with eGFR above the median [4]. A meta-analysis of studies assessing the relationship between heart failure and kidney dysfunction found that at least 63% of patients had mild kidney injury, and 20% had moderate or severe renal dysfunction [5]. Further, a consistent risk relationship of a 7% increase in mortality for every 10 ml/min decrease in eGFR was found. These findings were also confirmed in a larger cohort of patients with heart cessation [6]. Twenty-five percent of patients hospitalized for acute heart failure had significantly higher kidney dysfunction (eGFR less than 60 ml/min/1.73 m2). The use of diuretics may be one of the risk factors for kidney dysfunction at least in the initial phase of hospitalization for patients with heart failure [7, 8].
Patients with higher kidney dysfunction are associated with extended hospitalizations days, as well as a higher risk of short- and long-term cardiac failure. In the large-scale Acute Decompensated Heart Failure National Registry (ADHERE) consisting of 105,388 hospitalized visits of patients with acute decompensated heart failure in the United States of America, the best predictors of the outcome included both serum blood urea nitrogen (BUN) and creatinine [9]. This refers to the strong independent pre- and post-kidney function of the prognostic equivalence.
Data from an Ontario heart failure population derivation cohort in the Enhanced Feedback for Effective Cardiac Treatment (EFFECT) study identified that older age, low systolic blood pressure, high serum BUN, and low serum sodium were independent forecasters of outcome [10]. In addition, a recent study of patients with diastolic heart failure showed that kidney dysfunction is associated with heart failure [11, 12], where major predictors included kidney dysfunction, age, plasma sodium, anemia, and dementia. Thus, the presence of kidney dysfunction contributed at least 20% to the increase of heart failure-related mortality [13].
Various cross-sectional studies have shown that the Framingham risk equation is insufficient to predict the extent of CVD risk in subjects with CKD [14-16]. The non-traditional risk factors such as physical inactivity, body weight, and composition are not included in Framingham risk equations which may play an important role in promoting ischemic heart disease in subjects with CKD. Traditional risk factors such as hypertension, diabetes, dyslipidemia, smoking, and family history of premature heart disease may have a different risk relationship with cardiovascular disease in CKD as compared to the general population.
Community-based differences in acute kidney injury (AKI) and CKD have been studied and African Americans are found at higher risk of kidney failure [17, 18]. The black population shows a 16% higher eGFR compared with the non-black population in age and creatinine-matched study [19]. Due to this difference removing the race coefficient is suggested but this may also cause an underestimation of eGFR in the black population, with potential unintended consequences at the individual and population levels [20]. The reason for these differences is not fully understood and thus use of race in eGFR calculations has been limited [21]. There are significant ongoing efforts to address the eGFR calculations to predict the risk of kidney failure and to better understand the racial differences.
Diabetic kidney disease and cardiovascular disease
The early marker for diabetic kidney disease is microalbuminuria which is a predictive risk marker for CKD. Microalbuminuria also causes poor glucose control, dyslipidemia, and increased blood pressure compared with diabetic subjects without microalbuminuria [22, 23].
Cross-sectional studies show for type 2 diabetic patients a stronger association between microalbuminuria and CVD as well as surrogate measures, such as carotid intima-media thickness [24]. In addition, Left Ventricular Hypertrophy (LVH) [25, 26], as well as different clinical symptoms of CVD such as peripheral vascular disease [27] and coronary artery disease [26, 28] were associated with microalbuminuria. This correlation was found in both patients with type 1 and type 2 diabetes. Likewise, longitudinal studies demonstrated that an increase in microalbuminuria leads to worse CVD outcomes and all-cause mortality in diabetic patients [26, 29-33].
Dialysis and cardiovascular disease
CVD mediated mortality is up to 30 times higher in dialysis patients as compared to the general population [34]. After stratification for age, CVD mediated death remains 5-fold higher in dialysis patients than in the general population, even in the aged population [35-37]. Moreover, kidney dysfunction contributes to the pathophysiology of a cardiorenal syndrome, including anemia, uremic cardiomyopathy, fluid overload, and secondary hyperparathyroidism. However, the unique physiology of cardiovascular abnormalities in dialysis patients remains poorly understood. Altered lipid metabolism and accumulation of gut microbiota-derived uremic toxins like trimethylamine N-oxidase (TMAO), also affect the cardiovascular function following kidney failure [38].
Because of the low levels of erythropoietin in end-stage renal disease, patients undergoing dialysis become anemic which increases the risk of cardiovascular disease [39]. Anemia promotes ischemia in the heart due to a decrease in oxygen delivery which represents a classical risk factor for atherosclerosis [40-42]. A decrease in shear stress due to changes in the signaling mechanisms in the endothelium may also contribute to vascular injury [43].
Thickening of blood vessels in patients undergoing dialysis is caused by disturbed homeostasis in vitamin D, calcium, and phosphate levels as well as fibroblast growth factor 23 [44], and chronic myocardial ischemia is caused by underlying calcification and atherosclerotic changes in the cardiac vasculature [45]. Moreover, artificial tubing and dialyzer membranes cause platelet abnormalities, and dialysis itself promotes inflammation and thrombosis [46]. Previously used complement-activating cellulose filters in hemodialysis increased the risk for anaphylactoid reactions, which is today largely prevented, as significant advancements in dialyzer membrane technology have happened since the use of Wilhelm Kolff’s sausage casings started [47].
Sudden cardiac deaths are very common in patients undergoing dialysis and one of the factors/reasons for this may be the electrolyte imbalance [48]. Moreover, in each dialysis session, about 10-12 grams of amino acids are lost through the filter [49]. Prevention of malnutrition enhances the quality of life and extends the life span of dialysis patients [50]. Thus, there is a need for enhancement of the quality of life by early diagnosis and treatment of CKD patients undergoing dialysis [50]. Higher CVD-related mortality has been associated with patients undergoing peritoneal dialysis as compared to hemodialysis patients [51, 52].
Cardiovascular disease in kidney transplant recipients
As much as 50% of all-cause mortality in kidney transplant recipients is due to CVD [53-55]. The rate of CVD-mediated mortality is two times higher in transplant recipients as compared to the general population [34, 55]. Kidney transplant recipients have a lower risk for cardiovascular disease than patients on dialysis which may be related to both selection bias for those undergoing transplantation and the removal of hemodynamic and uremic abnormalities associated with dialysis in those transplant recipients. Also, age and sex-matched data show that CVD morbidity is higher in kidney transplant recipients than in the general population. The prevalence of left ventricle hypertrophy is up to 70% [56-58], and the incidence of CVD is at least 3 to 5 times higher in patients with kidney transplants as compared to non-transplanted patients, Additionally, the prevalence of coronary artery disease was increased by 15% in patients with kidney transplants as compared to the general population [34, 59].
Cardiovascular disease in nondiabetic kidney disease
Genetic considerations:
Irrespective of the pathophysiological mechanism of the underlying genetic disease, the overall risk for CVD is increased as compared to the general population. A registry of a cohort of 116 patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD) with a mean age of 41 years had a 41% prevalence of left ventricular hypertrophy, and 23% subjects had it without hypertension, using echocardiographic methods [60]. In a study of 31 ADPKD patients with hypertension, ADPKD patients had significantly greater left ventricle myocardial infarction. Intriguingly, right ventricular function, as assessed by the myocardial performance index, was significantly higher in both ADPKD patients and normotensive patients [61], and in ADPKD patients with well-preserved renal function.
Focal segmental glomerular sclerosis is the most common glomerular disease in children and adults leading to end-stage kidney disease and ultimately renal replacement therapy [62]. There is a multifactorial reason for mortality including inflammatory and immunological mechanisms in this disease, with mutations in the APOL1 gene which is associated with a higher risk of the disease and leads to increased CVD [63-66]. High-risk mutation known as G1 and G2 in the APOL1 gene is associated with the African American population. A recent study suggested that African American individuals, having the high-risk APOL1 variant gene show a steeper decline in renal function than individuals having low-risk variants [67]. Interestingly, G1 and G2 mutations of APOL1 seem to cause cellular injury specifically in podocytes [68, 69].
Reduced estimated glomerular filtration rate (eGFR)
Reduced eGFR is associated with a high prevalence of CVD risk factors and a higher prevalence of CVD surrogates and clinical CVD. For example, several studies across a broad spectrum of populations, such as the Framingham and Framingham Offspring Studies, the Atherosclerosis Risk In Communities (ARIC) Study, the Hypertension Optimal Treatment (HOT) Study, the HOPE study, and the Cardiovascular Health Study (CHS) have shown that levels of systolic blood pressure and total cholesterol and the percentage of subjects with low HDL cholesterol are greater in patients with decreased eGFR. In addition, the percentages of patients with diabetes, ischemic heart disease, and heart failure are higher in those with lower eGFR [70-72]. Recent studies have shown that kidney function is associated with the extent of angiographic coronary disease [73, 74].
Proteinuria
Non-diabetic subjects with microalbuminuria have a higher risk of CVD as compared to diabetic patients without microalbuminuria due to high blood pressure, and dyslipidemia [75-77]. Microalbuminuria is also related to the increased thickness of the intima and media of the carotid artery in patients with hypertension [78], and electrocardiographically signs of ischemia of the myocardium [79]. Clinical CVD has been found to be increased in patients with microalbuminuria as compared to patients without microalbuminuria [77].
Longitudinal studies demonstrated that proteinuric non-diabetic kidney disease patients have an increased risk of CVD as compared to proteinuric patients with diabetic kidney disease [29, 80-87]. Microalbuminuria in nondiabetic subjects in the HOPE study was associated with a 61% increased risk of the composite endpoint of stroke, myocardial infarction, or CVD death and a 2-fold increase in risk for all-cause mortality [29]. Microalbuminuria in nondiabetic patients may reflect generalized endothelial dysfunction [88-91] or abnormalities of the fibrinolytic and coagulation pathway, which may be a marker of the underlying inflammatory status [92] or may denote the greater severity of the target end-organ damage as opposed to diabetic patients.
Risk factors and pathophysiology for a Cardiorenal Syndrome (CRS)
The common primary cause for kidney dysfunction in the setting of heart failure or cardiac dysfunction is diabetes, hypertension, and underlying vascular diseases [93].
In general, older patients with a history of either heart failure, kidney failure, or both have higher mortality. The risk factors for diuretic resistance or kidney dysfunction in the setting of acute decompensated heart failure are not well described, but likely are also dominated by a similar risk factor.
The pathophysiology of a CRS likely varies according to the specific clinical conditions. Studies suggest that intra- and inter-renal hemodynamics such as systemic [94] and trans-renal perfusion pressure [95] including neurohormonal factors [96] are closely associated with a CRS. Likewise, an elevated central venous pressure was associated with an increased risk of mortality and AKI in critically ill adult patients hospitalized in the intensive care unit [97]. Therefore, reducing the central venous pressure can result in a significant enhancement in the blood flow of the kidney and ultimately increase the urine output.
Patients with diabetes and hypertension have a significant reduction in glomerular filtration, which further worsens any pre-existing kidney dysfunction. Activation of arterial baroreceptors and intra-renal sensors mediates the activation of neurohormonal factors. This activates intrinsic self-defence mechanisms to maintain blood pressure, and together with the intravascular volume that also activates the renin-angiotensin system, which was defined as the sympathoadrenal system. All these factors cause vasoconstriction in the kidney leading to decreased renal flow and low GFR. Subsequently, inflammatory cytokines are released because of hypoxia which leads finally to kidney dysfunction.
Recent reports indicate that the presence of a low-flow state does not explain the pathophysiology of CRS completely. The Acute Decompensated Heart Failure National Registry (ADHERE) showed that increased serum creatinine levels were similar among patients with acute heart failure and a reduced versus preserved systolic function [57]. Additionally, patients with evidence of acute CRS have preserved or elevated blood pressure and normal left ventricular ejection fraction [98]. Reduction in renal blood flow in patients with decompensated heart failure with relative preservation of their glomerular filtration rate was found [14]. The decrease in glomerular pressures and reduced GFR were driven by pre-glomerular vasoconstriction from extreme levels of RAAS and neurohormonal activation [99].
In this context adenosine is a factor that is related to the tubuloglomerular response. It is released by the kidney under stress and binds on the receptors of the afferent arterioles and causes vasoconstriction, which decreases blood flow in the kidney [100]. This pathway needs further attention to better understand the effects of blocking adenosine and its outcome on the resulting kidney function.
Clinical challenges in the management of patients with cardiorenal syndrome
Clinicians are facing increasing challenges in the treatment of patients with contradicting medication available for the two individual organs heart and kidney. For the kidney, it is important to regain its function by increasing the vascular volume by increasing the overall sodium load while salt on the other side will worsen the cardiac complications. In contrast, cardiologists treating a patient’s volume overload and cardiac congestion with aggressive use of diuretics will lead to a decrease in blood pressure which can cause acute kidney dysfunction. Not surprisingly, many patients end up being discharged from the hospital either still volume loaded or markedly worse in terms of renal function. Consequently, there is a high readmission rate for patients recently discharged from hospitals with heart failure or renal failure. Thus, separately nephrologists and cardiologists will often provide counsels that may be incompatible and thus need to know the underlying CVD and CKD, respectively.
Schematic of cross-talk between cardiac complications and kidney disease are shown in (Figure 1). 
Figure 1: Progression of chronic kidney disease leads to decrease in eGFR and increase in albuminuria. Decreased eGFR and increased albuminuria possess higher risk of cardiovascular disease.
Common strategies for treating the cardiorenal syndrome
Moving forward, the identification of CRS is important and long-term treatment plans should be given priority. There is a need for an optimized therapy that can preserve kidney function as well as heart function. Novel adenosine receptor blockers and other improved volume regulators that can preserve both kidney and cardiac function are much awaited to be developed.
The newer class of drugs targeting multiple symptoms
In recent years, inhibitors of Sodium-Glucose Co-Transporter 2 (SGLT2), Glucagon-Like Peptide-1 receptor (GLP1), and Dipeptidyl Peptidase IV (DPP4) inhibitors have been approved for patients with diabetes for glycemic control.
SGLT2 inhibitors: SGLT2 inhibitors decrease the risk of heart failure, hospitalizations, and serious kidney outcomes among patients with diabetes [101-103]. The cardio-renal benefits are so dramatic that they cannot be explained by the glucose-lowering action of SGLT2 inhibitors [104]. Hence, it is suggested that heart and kidney protection by SGLT2 inhibitors would be apparent in patients without diabetes as well [105, 106]. SGLT2 inhibition in patients with type 2 diabetes improved glomerular hemodynamic function, along with the reduced risk of CKD and CVD [107].
GLP1 inhibitors: The major pathways underlying the GLP-1 mechanism of action in the kidney are regulation of atrial natriuretic peptide and effects on the renin-angiotensin system. In Dahl salt-sensitive rats, infusion of GLP-1 increased the eGFR, urinary flow, and urinary sodium. GLP-1 decreases reactive oxygen species (ROS) production and inflammation in vivo and in vitro by increasing the GLP-1 receptor [108]. GLP-1 receptor agonists (RAs) increase insulin secretion and stimulate glucose uptake, leading to a reduction in glycated hemoglobin (HbA1c) [109]. In randomized controlled trials, GLP-1 RAs reduce systolic blood pressure by 1 to 2 mm Hg, with lesser effects on diastolic blood pressure [110]. In addition, GLP-1 RAs did not reduce the incidence of new-onset of hypertension [62] and further increased heart rate by 2 to 3 beats per minute [110]. In a trial with healthy obese men with exenatide administration, there was a 33% reduction in afferent arteriolar resistance and no change in efferent arteriolar resistance a single dose, thus increasing the blood flow and eGFR [111]. In addition, GLP-1 RAs reduce cardiovascular (CV) risk compared to placebo in cardiovascular outcome trials (CVOTs). GLP-1 RAs have only a modest effect on the risk of heart failure after hospitalization [112].
DPP4 inhibitors: DPP-4 inhibitors show protection from fibrosis, reduction in albuminuria, and inhibit the progression to advanced CKD. DPP4 inhibitor, linagliptin, decreases the expression of the advanced glycation end product (AGE) receptor which in turn lowers the oxidative stress and inflammation in mouse models. Thus, linagliptin leads to decreased progression of advanced diabetic kidney disease (DKD).
Although, multiple animal models show beneficial effects of linagliptin on kidney fibrosis, its translation into the patients with DKD to inhibit its progression is not affirmative. However, DPP-4 inhibitors offer good glycemic control in patients with DKD, and hypertension [113]. Saxagliptin, another DPP4 inhibitor in an assessment of vascular outcomes recorded in patients with diabetes thrombolysis in myocardial Infarction trial, showed an association with significant reductions in albuminuria [114]. However, a 2-year follow-up cohort did not show any difference in eGFR or the incidence of kidney endpoints, such as doubling of the serum creatinine values or kidney failure [113]. Similarly, sitagliptin, there was a significant improvement in albuminuria but no change in eGFR [115].
The primary result of the cardiovascular and renal microvascular outcome trial with linagliptin was a cardiovascular disease composite, including nonfatal myocardial infarction, cardiovascular death, and nonfatal stroke. Linagliptin trial showed a cardiovascular safety profile with no significant difference in kidney outcomes with a 40% decrease in eGFR [116]. Notably, patients randomized to receive linagliptin had less progression of albuminuria [36]. Thus, additional trials with longer follow-up times are warranted to be conducted to assess its effect on the progression of DKD.
Summary of the effects of DPP-4, GLP-1, and SGLT-2 inhibitors in cardiovascular disease, diabetic and diabetic kidney disease has been shown in (Figure 2). 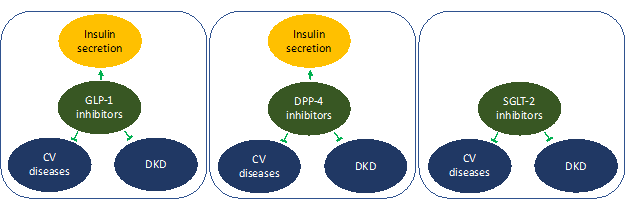
Figure 2: GLP-1 and DPP-4 and SGLT-2 inhibitors offers protection in cardiovascular and diabetic kidney disease. In addition, GLP-1 and DPP-4 inhibitors offers additional benefit for management of diabetes by improving insulin secretion
Open Questions
There are many remaining questions such as the effect of a low range decrease in eGFR on the development of CVD (89 to 60), and for instance if non-traditional risk factors and CKD at all stages is a risk factors for CVD. Also, how cellular remodeling of the left ventricle happens in patients with CKD. All of this should be further addressed to improve treatment strategies at an early stage of disease in affected patients.
Conflict of Interest
Authors declare no conflict of interests.
Authors Contributions
AKA conceptualized the manuscript, searched for the literature, and wrote the manuscript. LZ searched the literature and edited the manuscript. AW and LLH edited the manuscript. All the authors approved the manuscript.
Acknowledgements
AA is supported by a Career Development Grant from the American Heart Association (19CDA34780005). LZ is supported by the National Science Foundation, China.
References
1. Vidal-Petiot E, Greenlaw N, Kalra PR, et al. (2019) Chronic kidney disease has a graded association with death and cardiovascular outcomes in stable coronary artery disease: an analysis of 21,911 patients from the CLARIFY Registry. J Clin Med 9: 4. https://doi.org/10.3390/jcm9010004
2. Matsushita K, Coresh J, Sang Y, et al. (2015) Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 3: 514-525. https://doi.org/10.1016/S2213-8587(15)00040-6
3. Nerpin E, Ingelsson E, Risérus U, et al. (2011) The combined contribution of albuminuria and glomerular filtration rate to the prediction of cardiovascular mortality in elderly men. Nephrol Dial Transplant 26: 2820-2827. https://doi.org/10.1093/ndt/gfq848
4. Esmeijer K, Geleijnse JM, de Fijter JW, et al. (2018) Cardiovascular risk factors accelerate kidney function decline in post-myocardial infarction patients: The alpha omega cohort study. Kidney Int Rep 3: 879-888. https://doi.org/10.1016/j.ekir.2018.03.005
5. Smith GL, Lichtman JH, Bracken MB, et al. (2006) Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol 47: 1987-1996. https://doi.org/10.1016/j.jacc.2005.11.084
6. Hillege HL, Nitsch D, Pfeffer MA, et al. (2006) Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 113: 671-678. https://doi.org/10.1161/CIRCULATIONAHA.105.580506
7. Khan YH, Sarriff A, Adnan AS, et al. (2016) Mallhi, chronic kidney disease, fluid overload and diuretics: A complicated triangle. PLoS One 11: e0159335. https://doi.org/10.1371/journal.pone.0159335
8. Hunt JC, Maher FT (1966) Diuretic drugs in patients with impaired renal function. Am J Cardiol 17: 642-647. https://doi.org/10.1016/0002-9149(66)90400-0
9. Fonarow GC, Adams KF, Abraham WT, et al. (2005) Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 293: 572-580. https://doi.org/10.1001/jama.293.5.572
10. Lee DS, Austin PC, Rouleau JL, et al. (2003) Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 290; 2581-2587. https://doi.org/10.1001/jama.290.19.2581
11. Bhatia RS, Tu JV, Lee DS, et al. (2006) Outcome of heart failure with preserved ejection fraction in a population-based study. New Eng J Med 355: 260-269. https://doi.org/10.1056/NEJMoa051530
12. Owan TE, Hodge DO, Herges RM, et al. (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. New Eng J Med 355: 251-259. https://doi.org/10.1056/NEJMoa052256
13. Schrier RW (2006) Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol 47: 1-8. https://doi.org/10.1016/j.jacc.2005.07.067
14. Cheung AK, Sarnak MJ, Yan G, et al. (2000) Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int 58: 353-362. https://doi.org/10.1046/j.1523-1755.2000.00173.x
15. Longenecker JC, Coresh J, Powe NR, et al. (2002) Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol 13: 1918-1927. https://doi.org/10.1097/01.ASN.0000019641.41496.1E
16. Sarnak MJ, Coronado BE, Greene T, et al. (2002) Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol 57: 327-335. https://doi.org/10.5414/cnp57327
17. Hsu CY, McCulloch CE, Fan D, et al. (2007) Community-based incidence of acute renal failure. Kidney Int 72: 208-212. https://doi.org/10.1038/sj.ki.5002297
18. Feest TG, Round A, Hamad S (1993) Incidence of severe acute renal failure in adults: results of a community based study. BMJ 306: 481-483. https://doi.org/10.1136/bmj.306.6876.481
19. DeBoer MD, Filipp SL, Musani SK, et al. (2018) Metabolic Syndrome severity and risk of CKD and worsened GFR: The jackson heart study. Kidney Blood Press Res 43: 555-567. https://doi.org/10.1159/000488829
20. Poggio ED, Wang X, Greene T, et al. (2005) Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459-466. https://doi.org/10.1681/ASN.2004060447
21. Levey AS, Titan SM, Powe NR, et al. (2020) Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol 15: 1203-1212. https://doi.org/10.2215/CJN.12791019
22. Rutherford E, Talle MA, Mangion K, et al. (2016) Defining myocardial tissue abnormalities in end-stage renal failure with cardiac magnetic resonance imaging using native T1 mapping. Kidney Int 90: 845-852. https://doi.org/10.1016/j.kint.2016.06.014
23. Graham-Brown MP, March DS, Churchward DR, et al.,(2016) Novel cardiac nuclear magnetic resonance method for noninvasive assessment of myocardial fibrosis in hemodialysis patients. Kidney Int 90: 835-844. https://doi.org/10.1016/j.kint.2016.07.014
24. Mykka?nen L, Zaccaro DJ, O’Leary DH, et al. Microalbuminuria and carotid artery intima-media thickness in nondiabetic and NIDDM subjects. The insulin resistance atherosclerosis study (IRAS). Stroke 28: 1710-1716. https://doi.org/10.1161/01.STR.28.9.1710
25. Suzuki K, Kato K, Hanyu O, et al. (2001) Left ventricular mass index increases in proportion to the progression of diabetic nephropathy in Type 2 diabetic patients. Diabetes Res Clin Pract 54: 173-180. https://doi.org/10.1016/S0168-8227(01)00318-7
26. Stephenson JM, Kenny S, Stevens LK, et al. (1995) Proteinuria and mortality in diabetes: the WHO multinational study of vascular disease in diabetes. Diabet Med 12: 149-155. https://doi.org/10.1111/j.1464-5491.1995.tb00446.x
27. Howard BV, Lee ET, Cowan LD, et al. (1995) Coronary heart disease prevalence and its relation to risk factors in American Indians. The Strong Heart Study. Am J Epidemiol 142: 254-268. https://doi.org/10.1093/oxfordjournals.aje.a117632
28. Lee KU, Park JY, Kim SW, et al. (1995) Prevalence and associated features of albuminuria in Koreans with NIDDM. Diabetes Care 18: 793-799. https://doi.org/10.2337/diacare.18.6.793
29. Gerstein HC, Mann JF, Yi Q, et al. (2001) Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421-426. https://doi.org/10.1001/jama.286.4.421
30. C. E. Mogensen CE (1984) Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med 310: 356-360. https://doi.org/10.1056/NEJM198402093100605
31. Messent JW, Elliott TG, Hill RD, et al. (1992) Prognostic significance of microalbuminuria in insulin-dependent diabetes mellitus: a twenty-three year follow-up study. Kidney Int 41: 836-839. https://doi.org/10.1038/ki.1992.128
32. Valmadrid CT, Klein R, Moss SE, et al. (2000) The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med 160: 1093-1100. https://doi.org/10.1001/archinte.160.8.1093
33. Weiner DE, Park M, Tighiouart H, et al. (2019) Albuminuria and allograft failure, cardiovascular disease events, and all-cause death in stable kidney transplant recipients: A cohort analysis of the FAVORIT trial. Am J Kidney Dis 73: 51-61. https://doi.org/10.1053/j.ajkd.2018.05.015
34. Foley RN, Parfrey PS, Sarnak MJ, et al. (1998) Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112-119. https://doi.org/10.1053/ajkd.1998.v32.pm9820470
35. Chung SH, Han DC, Noh H, et al. (2010) Risk factors for mortality in diabetic peritoneal dialysis patients. Nephrol Dial Transplant 25: 3742-3748. https://doi.org/10.1093/ndt/gfq233
36. Locatelli F, Pozzoni P, Tentori F, et al. (2003) Epidemiology of cardiovascular risk in patients with chronic kidney disease. Nephrol Dial Transplant 18: Suppl 7, vii2-9. https://doi.org/10.1093/ndt/gfg1072
37. Foley RN, Murray AM, Li S, et al. (2005) Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489-495. https://doi.org/10.1681/ASN.2004030203
38. Heianza Y, Ma W, Manson JE, et al. (2017) Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies. J Am Heart Assoc 6: e004947. https://doi.org/10.1161/JAHA.116.004947
39. Foley RN, Parfrey PS, Harnett JD, et al. (1996) The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis 28: 53-61. https://doi.org/10.1016/S0272-6386(96)90130-4
40. Eckardt KU (2001) Anaemia in end-stage renal disease: pathophysiological considerations. Nephrol Dial Transplant 16: Suppl 7, 2-8. https://doi.org/10.1093/ndt/16.suppl_7.2
41. Boulanger CM, Amabile N, Gue?rin AP, et al. (2007) In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension 49: 902-908. https://doi.org/10.1161/01.HYP.0000259667.22309.df
42. Malyszko J (2010) Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta 411: 1412-1420. https://doi.org/10.1016/j.cca.2010.06.019
43. Patel TV, Mittal BV, Keithi-Reddy SR, et al. (2008) Endothelial activation markers in anemic non-dialysis chronic kidney disease patients. Nephron Clin Pract 110: c244-250. https://doi.org/10.1159/000167872
44. Ganidagli SE, Altunoren O, Erken E, et al. (2017) The relation between hemoglobin variability and carotid intima-media thickness in chronic hemodialysis patients. Int Urol Nephrol 49: 1859-1866. https://doi.org/10.1007/s11255-017-1651-6
45. Herzog CA (2005) Sudden cardiac death and acute myocardial infarction in dialysis patients: perspectives of a cardiologist. Semin Nephrol 25: 363-366. https://doi.org/10.1016/j.semnephrol.2005.05.003
46. Szczech LA, Barnhart HX, Inrig JK, et al. (2008) Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 74: 791-798. https://doi.org/10.1038/ki.2008.295
47. Nilsson B, Ekdahl KN, Mollnes TE, et al. (2007) The role of complement in biomaterial-induced inflammation. Mol Immunol 44: 82-94. https://doi.org/10.1016/j.molimm.2006.06.020
48. Genovesi S, Boriani G, Covic A, et al. (2019) Sudden cardiac death in dialysis patients: different causes and management strategies. Nephrol Dial Transplant 36: 396-405. https://doi.org/10.1093/ndt/gfz182
49. Sabatino A, Regolisti G, Karupaiah T, et al. (2017) Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin Nutr 36: 663-671. https://doi.org/10.1016/j.clnu.2016.06.007
50. Günalay S, Öztürk YK, Akar H, et al. (2018) The relationship between malnutrition and quality of life in haemodialysis and peritoneal dialysis patients. Rev Assoc Med Bras 64: 845-852. https://doi.org/10.1590/1806-9282.64.09.845
51. Bloembergen WE, Port FK, Mauger EA, et al. (1995) A comparison of cause of death between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 6: 184-191. https://doi.org/10.1681/ASN.V62184
52. Wang IK, Lu CY, Lin CL, et al. (2016) Comparison of the risk of de novo cardiovascular disease between hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Int J Cardiol 218: 219-224. https://doi.org/10.1016/j.ijcard.2016.05.036
53. Dimeny EM (2002) Cardiovascular disease after renal transplantation. Kidney Int Suppl 61: Suppl 80, S78-S84. https://doi.org/10.1046/j.1523-1755.61.s80.14.x
54. Ojo AO, Hanson JA, Wolfe RA, et al. (2000) Long-term survival in renal transplant recipients with graft function. Kidney Int 57: 307-313. https://doi.org/10.1046/j.1523-1755.2000.00816.x
55. Lindholm AN, Albrechtsen DA, Frödin LA, et al. (1995) Ischemic heart disease--major cause of death and graft loss after renal transplantation in Scandinavia. Transplantation 60: 451-457. https://doi.org/10.1097/00007890-199509000-00008
56. Parfrey PS, Harnett JD, Foley RN, et al. (1995) Impact of renal transplantation on uremic cardiomyopathy. Transplantation 60: 908-914.
57. Hernández D, Lacalzada J, Rufino M, et al. (1997) Prediction of left ventricular mass changes after renal transplantation by polymorphism of the angiotensin-converting-enzyme gene. Kidney Int 51: 1205-1211. https://doi.org/10.1038/ki.1997.164
58. Huting J (1992) Course of left ventricular hypertrophy and function in end-stage renal disease after renal transplantation. Am J Cardiol 70: 1481-1484.
59. Kasiske BL (1988) Risk factors for accelerated atherosclerosis in renal transplant recipients. Am J Med 84: 985-992. https://doi.org/10.1016/0002-9343(88)90302-6
60. Chapman AB, Johnson AM, Rainguet S, et al. (1997) Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 8: 1292-1297. https://doi.org/10.1681/ASN.V881292
61. Oflaz H, Alisir S, Buyukaydin B, et al. (2005) Biventricular diastolic dysfunction in patients with autosomal-dominant polycystic kidney disease. Kidney Int 68: 2244-2249. https://doi.org/10.1111/j.1523-1755.2005.00682.x
62. Korbet SM, Schwartz MM, Lewis EJ (1994) Primary focal segmental glomerulosclerosis: clinical course and response to therapy. Am J Kidney Dis 23: 773-783. https://doi.org/10.1016/S0272-6386(12)80128-4
63. Ito K, Bick AG, Flannick J, et al. (2014) Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res 114: 845-850. https://doi.org/10.1161/CIRCRESAHA.114.302347
64. Ng DK, Robertson CC, Woroniecki RP, et al. (2017) APOL1-associated glomerular disease among African-American children: a collaboration of the chronic kidney disease in children (CKiD) and nephrotic syndrome study network (NEPTUNE) cohorts. Nephrol Dial Transplant 32: 983-990. https://doi.org/10.1093/ndt/gfw061
65. Woroniecki RP, Ng DK, Limou S, et al. (2016) Renal and cardiovascular morbidities associated with APOL1 status among african-american and non-african-american children with focal segmental glomerulosclerosis. Front Pediatr 4: 122. https://doi.org/10.3389/fped.2016.00122
66. Parsa A, Kao WL, Xie D, et al. (2013) APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369, 2183-2196. https://doi.org/10.1056/NEJMoa1310345
67. Hayek SS, Koh KH, Grams ME, et al. (2017) A tripartite complex of suPAR, APOL1 risk variants and alphavbeta3 integrin on podocytes mediates chronic kidney disease. Nat Med 23: 945-953. https://doi.org/10.1038/nm.4362
68. Fu Y, Zhu JY, Richman A, et al. (2017) APOL1-G1 in nephrocytes induces hypertrophy and accelerates cell death. J Am Soc Nephrol 28: 1106-1116. https://doi.org/10.1681/ASN.2016050550
69. Kruzel-Davila E, Shemer R, Ofir A, et al. (2017) APOL1-mediated cell injury involves disruption of conserved trafficking processes. J Am Soc Nephrol 28: 1117-1130. https://doi.org/10.1681/ASN.2016050546
70. Culleton BF, Larson MG, Wilson PW, et al. (1999) Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int 56: 2214-2219. https://doi.org/10.1046/j.1523-1755.1999.00773.x
71. Manjunath G, Tighiouart H, Coresh J, et al. (2003) Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 63: 1121-1129. https://doi.org/10.1046/j.1523-1755.2003.00838.x
72. Manjunath G, Tighiouart H, Ibrahim H, et al. (2003) Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol 41; 47-55. https://doi.org/10.1016/s0735-1097(02)02663-3
73. Cai Q, K Mukku V, Ahmad M (2013) Coronary artery disease in patients with chronic kidney disease: a clinical update. Curr Cardiol Rev 9: 331-339. https://doi.org/10.2174/1573403x10666140214122234
74. Sarnak MJ, Amann K, Bangalore S, et al. (2019) Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol 74: 1823-1838. https://doi.org/10.1016/j.jacc.2019.08.1017
75. Pontremoli R, Sofia A, Ravera M, et al. (1997) Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: A genoa investigation on complications. Hypertension 30: 1135-1143. https://doi.org/10.1161/01.HYP.30.5.1135
76. Dell'Omo G, Penno G, Giorgi D, et al. (2002) Association between high-normal albuminuria and risk factors for cardiovascular and renal disease in essential hypertensive men. Am J Kidney Dis 40: 1-8. https://doi.org/10.1053/ajkd.2002.33906
77. Hillege HL, Janssen WM, Bak AA, et al. (2001) Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 249: 519-526. https://doi.org/10.1046/j.1365-2796.2001.00833.x
78. Bigazzi R, Bianchi S, Nenci R, et al. (1995) Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 9: 827-833.
79. Diercks GF, Hillege HL, van Boven AJ, et al. (2001) Relation between albumin in the urine and electrocardiographic markers of myocardial ischemia in patients without diabetes mellitus. Am J Cardiol 88: 771-774. https://doi.org/10.1016/S0002-9149(01)01849-5
80. RIS RF, Agewall S, Wikstrand J, et al. (1997) Usefulness of microalbuminuria in predicting cardiovascular mortality in treated hypertensive men with and without diabetes mellitus. Risk Factor Intervention Study Group. Am J Cardiol 80: 164-169. https://doi.org/10.1016/S0002-9149(97)00312-3
81. Miettinen H, Haffner SM, Lehto S, et al. (1996) Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non-insulin-dependent diabetic subjects. Stroke 27: 2033-2039. https://doi.org/10.1161/01.STR.27.11.2033
82. De Leeuw PW, Thijs L, Birkenhäger WH, et al. (2002) Prognostic significance of renal function in elderly patients with isolated systolic hypertension: results from the Syst-Eur trial. J Am Soc Nephrol 13: 2213-2222. https://doi.org/10.1097/01.ASN.0000027871.86296.92
83. Grimm Jr RH, Svendsen KH, Kasiske B, et al. (1997) Proteinuria is a risk factor for mortality over 10 years of follow-up. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Kidney Int Suppl 63: S10-14.
84. Hillege HL, Fidler V, Diercks GF, et al. (2002) Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777-1782. https://doi.org/10.1161/01.CIR.0000031732.78052.81
85. Muntner P, He J, Hamm L, et al. (2002) Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13: 745-753. https://doi.org/10.1681/ASN.V133745
86. Kuusisto J, Mykka?nen L, Pyo?ra?la? K, et al. (1995) Hyperinsulinemic microalbuminuria. A new risk indicator for coronary heart disease. Circulation 91: 831-837. https://doi.org/10.1161/01.CIR.91.3.831
87. Yudkin J, Forrest R, Jackson C (1988) Microalbuminuria as predictor of vascular disease in non-diabetic subjects. Islington Diabetes Survey. Lancet 2: 530-533. https://doi.org/10.1016/S0140-6736(88)92657-8
88. Pedrinelli R, Dell’Omo G, Penno G, et al. (2001) Non-diabetic microalbuminuria, endothelial dysfunction and cardiovascular disease. Vasc Med 6: 257-264. https://doi.org/10.1177/1358836X0100600410
89. Clausen P, Feldt-Rasmussen B, Jensen G, et al. (1999) Endothelial haemostatic factors are associated with progression of urinary albumin excretion in clinically healthy subjects: a 4-year prospective study. Clin Sci (Lond) 97: 37-43. https://doi.org/10.1042/cs0970037
90. Jensen JS (1995) Renal and systemic transvascular albumin leakage in severe atherosclerosis. Arterioscler Thromb Vasc Biol 15: 1324-1329. https://doi.org/10.1161/01.ATV.15.9.1324
91. Nerpin E, Ingelsson E, Risérus U, et al. (2012) Association between glomerular filtration rate and endothelial function in an elderly community cohort. Atherosclerosis 224: 242-246. https://doi.org/10.1016/j.atherosclerosis.2012.07.008
92. Paisley KE, Beaman M, Tooke JE, et al. (2003) Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int 63: 624-633. https://doi.org/10.1046/j.1523-1755.2003.00768.x
93. Forman DE, Butler J, Wang Y, et al. (2004) Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43: 61-67. https://doi.org/10.1016/j.jacc.2003.07.031
94. Lindenfeld J, Albert NM, Boehmer JP, et al. (2010) HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail 16: e1-194. https://doi.org/10.1016/j.cardfail.2010.04.004
95. Vinod P, Krishnappa V, Chauvin AM, et al. (2017) Cardiorenal syndrome: Role of arginine vasopressin and vaptans in heart failure. Cardiol Res 8: 87-95. https://dx.doi.org/10.14740/cr553w
96. Rangaswami J, Vivek B, Blair JE, et al. (2019) Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the American heart association. Circulation 139: e840-e878. https://doi.org/10.1161/CIR.0000000000000664
97. Chen CY, Zhou Y, Wang P, et al. (2020) Elevated central venous pressure is associated with increased mortality and acute kidney injury in critically ill patients: a meta-analysis. Crit Care 24: 80. https://doi.org/10.1186/s13054-020-2770-5
98. Peteiro J, Alvarez N, Calvino R, et al. 91994) Changes in left ventricular mass and filling after renal transplantation are related to changes in blood pressure: an echocardiographic and pulsed Doppler study. Cardiology 85: 273-283. https://doi.org/10.1159/000176695
99. Warram JH, Gearin G, Laffel L, et al. (1996) Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol 7: 930-937. https://doi.org/10.1681/asn.v76930
100. Gottlieb SS, Brater DC, Thomas I, et al. (2002) BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation 105: 1348-1353. https://doi.org/10.1161/hc1102.105264
101. Zelniker TA, Wiviott SD, Raz I, et al. (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393: 31-39. https://doi.org/10.1016/S0140-6736(18)32590-X
102. Becher PM, Schrage B, Ferrannini G, et al. (2020) Use of sodium-glucose co-transporter-2 inhibitors in patients with and without type 2 diabetes: implications for incident and prevalent heart failure. Eur J Heart Fail 22: 604-617. https://doi.org/10.1002/ejhf.2131
103. Neuen BL, Young T, Heerspink HJ, et al. (2019) SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 7: 845-854. https://doi.org/10.1016/S2213-8587(19)30256-6
104. Fernandez-Fernandez B, Sarafidis P, Kanbay M, et al. (2020) SGLT2 inhibitors for non-diabetic kidney disease: drugs to treat CKD that also improve glycaemia. Clin Kidney J 13: 728-733. https://doi.org/10.1093/ckj/sfaa198
105. Packer M (2020) SGLT2 Inhibitors Produce Cardiorenal Benefits by Promoting Adaptive Cellular Reprogramming to Induce a State of Fasting Mimicry: A Paradigm Shift in Understanding Their Mechanism of Action. Diabetes Care 43: 508-511. https://doi.org/10.2337/dci19-0074
106. McMurray JJ, Solomon SD, Inzucchi SE, et al. (2020) Dapagliflozin in patients with heart failure and reduced ejection fraction. Reply. New Eng J Med 382: 973. https://doi.org/10.1056/nejmc1917241
107. Tuttle KR, Brosius III FC, Cavender MA, et al. (2020) SGLT2 inhibition for CKD and cardiovascular disease in type 2 diabetes: Report of a scientific workshop sponsored by the national kidney foundation. Am J Kidney Dis 70: 1-16. https://doi.org/10.2337/dbi20-0040
108. Coppolino G, Leporini C, Rivoli L, et al. (2018) Exploring the effects of DPP-4 inhibitors on the kidney from the bench to clinical trials. Pharmacol Res 129: 274-294. https://doi.org/10.1016/j.phrs.2017.12.001
109. Maiorino MI, Chiodini P, Bellastella G, et al. (2017) Insulin and glucagon-like peptide 1 receptor agonist combination therapy in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Care 40: 614-624. https://doi.org/10.2337/dc16-1957
110. Sun F, Wu S, Guo S, et al. (2015) Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Res Clin Pract 110: 26-37. https://doi.org/10.1016/j.diabres.2015.07.015
111. Muskiet MH, Tonneijck L, Smits MM, et al. (2016) Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes Metab 18: 178-185. https://doi.org/10.1111/dom.12601
112. Kristensen SL, Rørth R, Jhund PS, et al. (2019) Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 7: 776-785. https://doi.org/10.1016/S2213-8587(19)30249-9
113. Mosenzon O, Leibowitz G, Bhatt DL, et al. (2017) Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 Trial. Diabetes Care 40: 69-76. https://doi.org/10.2337/dc16-0621
114. Laakso M, Rosenstock J, Groop PH, et al. (2015) Treatment with the dipeptidyl peptidase-4 inhibitor linagliptin or placebo followed by glimepiride in patients with type 2 diabetes with moderate to severe renal impairment: a 52-week, randomized, double-blind clinical trial. Diabetes Care 38: e15-17. https://doi.org/10.2337/dc14-1684
115. Cornel JH, Bakris GL, Stevens SR, et al. (2016) Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care 39: 2304-2310. https://doi.org/10.2337/dc16-1415
116. Rosenstock J, Perkovic V, Johansen OE, et al. (2019) Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: The CARMELINA randomized clinical trial. JAMA 321: 69-79. https://doi.org/10.1001/jama.2018.18269
