Cardiac Resynchronization Therapy delivered Using Left Bundle Branch Pacing: Are we now Ready for Randomized Control Trials?
Authors: Fengwei Zou1, Zachary I. Whinnett2, Jiangang Zou3*
1Montefiore Medical Center, Bronx, New York, USA
2National Heart and Lung Institute, Imperial College London, United Kingdom
3Department of Cardiology, First Affiliated Hospital, Nanjing Medical University, Nanjing, China
*Correspondence to: Professor. Jiangang Zou, MD, PhD, FHRS. Department of Cardiology, First Affiliated Hospital, Nanjing Medical University, Nanjing, China. Email address: jgzou@njmu.edu.cn
Received: 16 October 2022; Accepted: 25 October 2022; Published: 29 October 2022
Citation: Fengwei Zou, Zachary I. Whinnett and Jiangang Zou (2022) Cardiac Resynchronization Therapy delivered Using Left Bundle Branch Pacing: Are we now Ready for Randomized Control Trials? 21st Century Cardiol, Volume 2 (5): 126
Abstract
Data from large randomized control trials has demonstrated that Cardiac resynchronization therapy (CRT) delivered using biventricular pacing (BiVP) is an effective therapy for patients with heart failure (HF) and LBBB. Left bundle branch pacing (LBBP) is a novel alternative method for delivering CRT, which can restore physiological left ventricular activation by engaging the left conduction system. Recent observational studies suggest that LBBP is feasible and safe, increases left ventricular ejection fraction and improves clinical outcomes in HF patients. LBBP-CRT has the potential to deliver more effective ventricular resynchronization than BiVP-CRT. LBBP may also be used in patients with pacing induced cardiomyopathy, LV lead failure, or no response to BiVP-CRT. The growing body of data from observational studies in now strong enough justify randomized controlled trials to establish whether CRT delivered using LBBP should become first line therapy.
Keywords:
Cardiac resynchronization therapy; Biventricular pacing; Left bundle branch pacing; Heart failure; Left bundle branch block
Introduction
Cardiac resynchronization therapy (CRT) delivered using biventricular pacing (BiVP), with a lead positioned in the coronary sinus, is an evidence-based therapy for heart failure patients with cardiomyopathy, reduced left ventricular (LV) ejection fraction (EF) and left bundle branch block (LBBB) [1,2]. However, about 30%-40% patients do not respond to CRT [3]. Recently, His-Purkinje conduction system pacing including His bundle pacing (HBP) and left bundle branch pacing (LBBP) have emerged as new physiological pacing modalities, which can preserve or recover cardiac synchrony by correcting LBBB [4-7]. Several studies using HBP have shown improved cardiac function in patients with reduced LVEF and LBBB [6,8-9]. However, HBP may require high pacing outputs to correct LBBB and implant success rates have been observed to be low in some studies. Huang W, et al. (2017) described a transeptal technique for delivering LBBP. They reported that this pacing approach, can correct LBBB in patients with non-ischemic cardiomyopathy (NICM) with low and stable pacing thresholds, and may even result in CRT super-response [7]. By targeting the more distal conduction system, LBBP has the potential to deliver higher LBBB correction rates than HBP [10] (Figure 1).
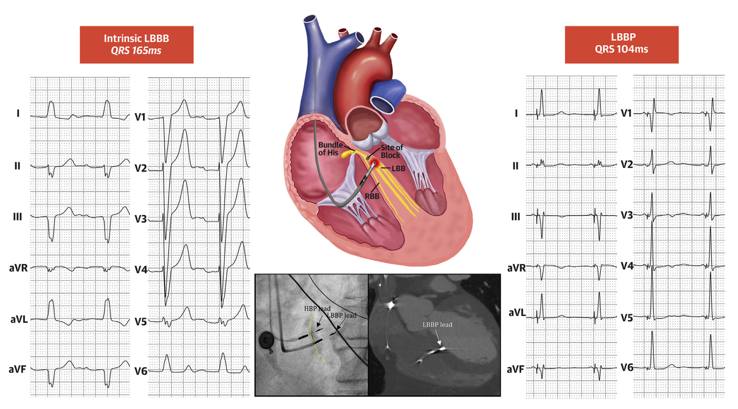
Figure 1: Correction of LBBB pattern with LBBP.
Twelve lead ECG at baseline and during LBBP are shown. QRS duration decreased from 165ms to 104ms. LBBP achieves capture of the left conduction system distal to the site of left bundle branch conduction block (red block) resulting in electrical resynchronization. Sheath angiography during implantation and computed tomography or echocardiography after implantation confirmed the depth of LBBP lead. Adapted from Huang W. et al. JACC Clin Electrophysiol. 2020 Jul;6(7):849-858 [10].
There is a growing body of evidence from observational studies that LBBP-CRT is technically feasible in many patients [11-12]. By directly capturing the left bundle it is often possible to restore normal physiological left ventricular activation in patients with LBBB or AV block. LBBP-CRT may therefore deliver more effective ventricular resynchronization compared to BiVP-CRT, which means that it has the potential to result in improved outcomes.
To date there has only been one randomized controlled trial (RCT) directly comparing LBBP-CRT with BiVP-CRT [13]. In the LBBP-RESYNC study, patients with NICM and LBBB were randomized to either LBBP-CRT or BiVP-CRT. The LBBP-CRT group obtained a greater improvement in LVEF, decrease of left ventricular end-systolic volume (LVESV) and N-terminal pro-B-type natriuretic peptide (NT-proBNP), and shortening of QRS duration after 6-months of treatment (Figure 2). No large-scale RCTs have been reported yet to confirm its long-term clinical efficacy in HF patients.
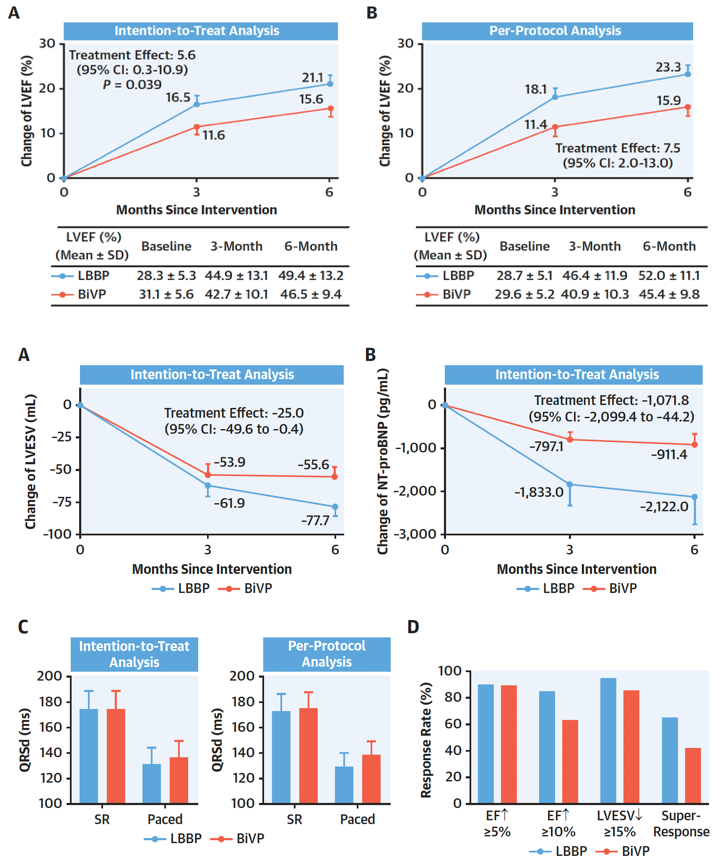
Figure 2: The primary and secondary endpoints of LBBP-RESYNC study.
The upper panel showed the primary endpoint: the mean differences in LVEF improvement adjusted by using a mixed-effects model for repeated measures from baseline to 3, 6 months after intervention by intention-to-treat analysis (A) and per-protocol analysis (B). The points represent the mean change of LVEF from baseline to 3 or 6 months after device implantation. Error bars represent the standard error of least-squares mean. The treatment effect is defined as the difference in changes of LVEF improvement between LBBP and BiVP groups. The lower panel showed the secondary endpoints: The mean differences in LVESV reduction and NT-proBNP decrease adjusted by using mixed-effects model for repeated measures from baseline to 6 months after the procedure by intention-to-treat analysis (A and B). The paced QRSd by intention-to-treat analysis and per-protocol analysis (C). The echocardiographic response rates, defined as LVEF improved ≥ 5% and 10% or LVESV reduction ≥ 15%, respectively, and super response rate, defined as both LVEF improvement >15% and LVEF >50% at 6-month follow-up between the 2 groups (D). Adapted from Wang Y. et al., J Am Coll Cardiol.2022 Sep 27;80(13):1205-1216 [13].
Electrophysiological Mechanisms of BiVP-CRT and LBBP-CRT in HF
When BiVP is delivered to patients with LBBB, who have very delayed left ventricular activation, it allows LV activation to be advanced and ventricular activation time to be reduced. However, BiVP only produces relatively modest reductions in ventricular activation time because propagation of the activation wavefront occurs via slow cell-to-cell activation. Indeed, it appears that AV shortening, rather than ventricular resynchronization is the dominant mechanism of acute hemodynamic benefit for BiVP-CRT in patients with SR and LBBB [14]. BiVP does not restore normal physiological ventricular activation times or patterns, which means that there may be significant missed potential when it is used as a method for improving cardiac function in patients with electrical conduction disease and heart failure. As result there has been a drive to develop alternative pacing approaches which deliver more effective ventricular resynchronization (Figure 3).
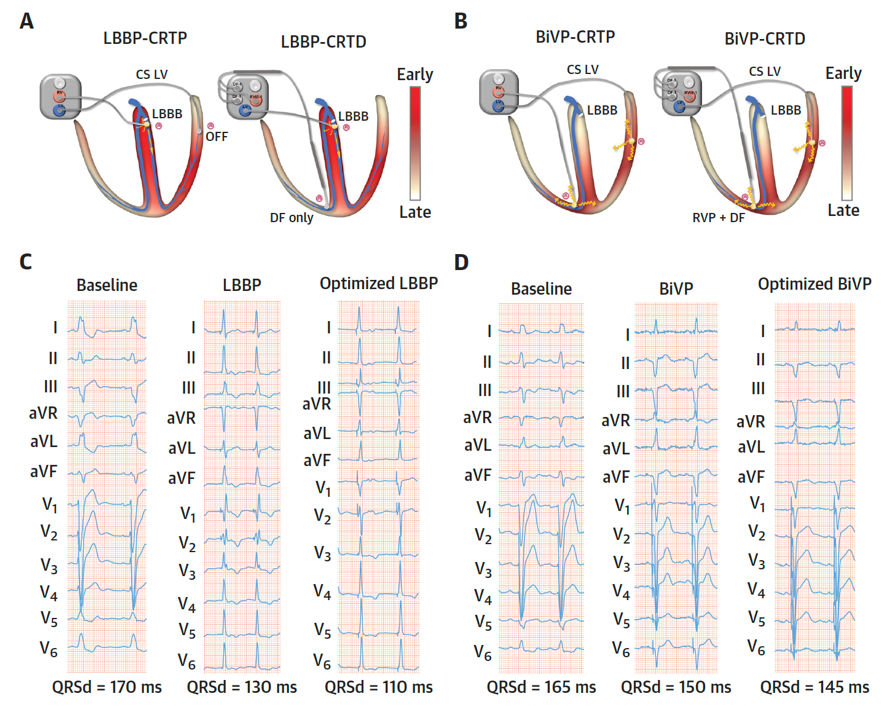
Figure 3: The different electrophysiological mechanisms of LBBP and BiVP for CRT in HF patients with LBBB.
The LV activation propagation in LBBP-CRT (A) and BiVP-CRT (B). Example of 12-lead electrocardiogram morphology and duration at baseline and after LBBP/BiVP with or without optimization (C and D). Adapted from Wang Y. et al., J Am Coll Cardiol.2022 Sep 27;80(13):1205-1216 [13].
By directly targeting the conduction system beyond the site of block, and capturing the fast conducting intrinsic conduction pathways, LBBP may restore normal physiological left ventricular activation patterns. LBBP-CRT often produces a narrower paced QRS duration compared to BiVP (Figure 3), suggesting that it delivers more effective left ventricular resynchronization.
The physiological advantages of LBBP, when it is used as a CRT strategy for patients who meet traditional BiVP-CRT indications, make it an exciting candidate to deliver the missed potential of BiVP-CRT.
Current Applications of LBBP in HF
1. LBBP in HF patients with reduced LVEF and LBBB
The first report of the use of left bundle pacing as a method for delivering CRT was in 2019, by Zhang W, et al. (2019) [15]. In 11 patients with HF and LBBB, they demonstrated that LBBP was technically feasible and significantly shortened QRS duration and left ventricular activation time (LVAT). After a mean of follow-up period of 6.7 months, New York Heart Association (NYHA) class, plasma level of NT-proBNP and LVEF significantly improved. Huang W, et al. (2020) [10] reported a prospective multicenter observational study of LBBP in patients with NICM with an LVEF less than 50% and LBBB. LBBP was successfully delivered in 61 of 63 patients. QRS duration narrowed from 169±16ms to 118±12ms, and LVEF increased significantly from 33±8% to 55±10%, with close to a 50% reduction in LVESV (123±61ml vs. 67±39ml). LVEF normalized (≥ 50%) in 75% of patients and NYHA class improved significantly from 2.8±0.6 to 1.4±0.6. Pacing thresholds and R-wave amplitudes remained stable during follow-up. Vijayaraman P, et al. (2021) [11] reported an international, retrospective, multicenter, cohort study designed to evaluate the real-world experience of LBBAP in CRT-eligible patients as either a first line approach or in patients in whom there had been failure to implant a BiVP. The population included in this study, was more diverse than the Huang W, et al. (2020) study: ischemic cardiomyopathy (ICM) in 44%, LBBB in 39% and non-LBBB in 46%. LBBAP-CRT was successfully achieved in 85% of patients (277/325) in this study. LBBAP resulted in both significant QRS narrowing from 152±32ms to 137±22ms and significant LVEF improvement from 33±10% to 44±11%. Interestingly, patients with LBBB and/or NICM had greater reductions in QRS duration and LVEF improvement compared with those with non-LBBB and/or ICM. Echocardiographic response rates were also greater in patients with LBBB compared with those with non-LBBB (87% vs. 67%). Super-response rates were also higher in patients with NICM compared with ICM (41% vs. 18%).
More recently, Chen X, et al. (2022) [12] compared the efficacy of LBBP-CRT with BiVP-CRT in HF patients with LVEF<35% and LBBB in a prospective multicenter non-randomized observational study. LBBP-CRT and BiVP-CRT were successful in 98% and 92% patients. LVEF increased in both groups without significant difference. However, a higher super-response rate was observed in LBBP-CRT vs BiVP-CRT at 6 months (53.06% vs. 36.59%, P = 0.016) and 12 months (61.22% vs. 39.22%, P=0.028). In 2022, Vijayaraman P, et al. (2021) [16] compared conduction system pacing (CSP) including HBP and LBBAP with BiVP in 477 CRT-indicated patients with LVEF<35%. Paced QRS duration in CSP was significantly narrower than BiVP (133±21ms vs 153±24ms). LVEF improved in both groups during mean follow-up of 27±12 months and was significantly greater with CSP compared to BiVP (39.7±13% vs 33.1±12%). The composite primary outcome of death or HF hospitalization was significantly lower with CSP compared to BiVP (28.3% vs 38.4%) irrespective of whether LBBB was present or not. Therefore, the results from observational studies suggest that LBBP-CRT is a very promising alternative to BiVP-CRT in patients with LBBB and severe left ventricular impairment. Randomized control trials are now required to confirm these findings.
2. LBBP in HF patients with mildly reduced LVEF and LBBB
With increasing evidence in HF management, HF with mildly reduced/mid-range LVEF has distinguished itself from the conventional dichotomy of HF categories. A small observational study (n=36) [17] reported the clinical outcomes of LBBAP in HF patients with LBBB and LVEF ≤ 35% vs 35-50%. While QRS duration reduced significantly in both groups, the hyper-responders (functional recovery plus LVEF ≥ 50%) to LBBAP in the LVEF 35-50% group was almost twice that seen in the LVEF ≤ 35% group (52.9% vs 33.3%). Patients in the LVEF 35-50% group also had significantly lower risk of HF hospitalizations or any-cause death. In one international registry study [11], thirty-two percent of patients had baseline LVEF between 36% and 50%. These patients achieved similar improvement of LVEF (42±5% to 50±8%) compared to those with LVEF <35% (27±7% to 40±11%) after LBBAP. It is reasonable to hypothesize that LBBP might also be an effective treatment for HF patients with mildly reduced LVEF and LBBB, but randomized studies are required to confirm this.
3. LBBP in patients with pacing induced cardiomyopathy (PICM)
Patients who require high ventricular pacing percentages are at risk of developing pacing induced cardiomyopathy. LBBP has the potential of mitigating PICM by capturing the physiological conduction system and restoring physiological ventricular activation. The feasibility and safety of LBBP upgrade in patients with PICM and infranodal atrioventricular block (AVB) has been reported. In one study, LBBP upgrade was successful in 19 of 20 patients [18]. At 12-month follow up, QRS duration significantly shortened (176.2±21.4ms to 120.9±15.2ms), LVEF significantly increased (36.3±6.5% to 51.9%±13.0%), and LVESV reduced from 180.1±43.5ml to 136.8±36.7ml while capture threshold remained stable during follow up. Qian Z, et al. (2021) [19] also reported LBBP upgrade in 27 patients with PICM. Similarly, QRS duration significantly shortened from 173.1±17.7ms to 117.5±10.2ms and LVEF increased from 40.3±5.2% to 48.1±9.5% at follow-up. Serum NT-proBNP levels decreased and NYHA classification also improved after LBBP upgrade. No upgrade-related complications were observed. Therefore, these findings from observational studies suggest that LBBP upgrade may be a feasible and effective approach in patients with PICM. The OptimPacing study is an ongoing multicenter RCT (NCT04624763) aiming to establish whether LBBP can preserve cardiac function and prevent PICM in patients with atrioventricular nodal block, compared to traditional right ventricular pacing.
4. LBBP in non-responders to BiVP-CRT and LV lead implant failures
Vijayaraman P, et al. (2022) [20] reported the feasibility and outcomes of rescue LBBAP in patients who either had failed LV lead implantation via the coronary sinus or were non-responders to BiVP-CRT in an international observational study. LBBAP was successfully performed in 200 patients including 156 LV lead implantation failures and 44 CRT non-responders. During mean follow-up of 12±10.1 months, LBBAP resulted in significant QRS narrowing from 170±28ms to 139±25ms with LVAT of 85±17ms and LVEF improved from 29%±10% to 40%±12%. However, the significant improvement in these clinical endpoints in the combined analysis was mostly driven by patients with LV lead failure rather than non-responders. Similarly, the risk of death or HF hospitalization was also lower in LV lead failure patients rather than BiVP non-responders (Figure 4). Therefore, this study supports the role of LBBP as an alternative to BiVP in patients in whom LV lead implantation is not feasible. Whether LBBP is beneficial in BiVP-CRT non-responders remains uncertain and requires further investigation. Close attention will need to be paid to patient selection when designing these studies.
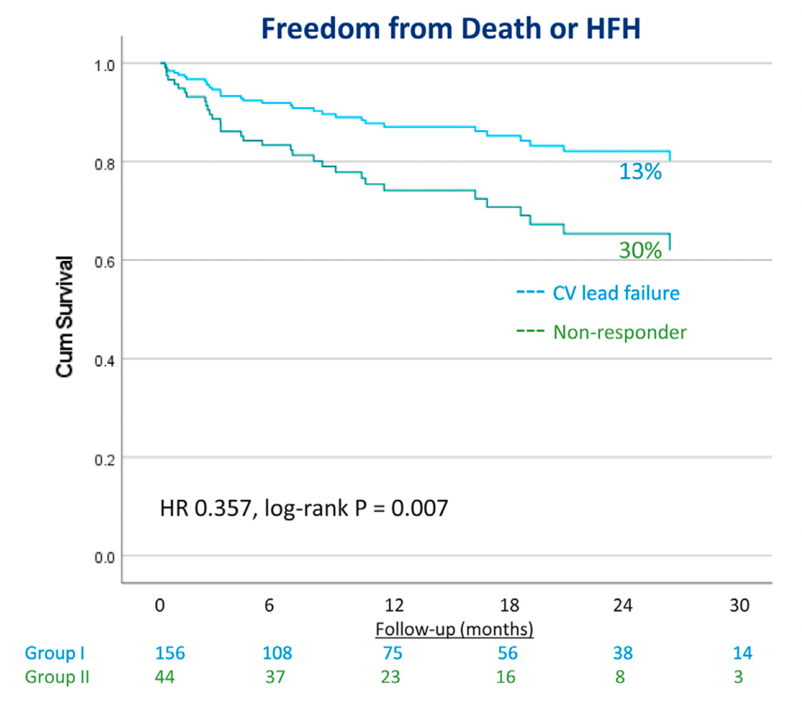
Figure 4: The comparison of freedom from death or HF hospitalization between CV lead failure and biventricular pacing non-responders by LBBAP.
Cox proportional hazard survival analysis showed significant reduction in the risk of death or HF hospitalization in patients with CV lead failure compared to biventricular pacing non-responders. HR=hazard ratio. Adapted from Vijayaraman P, et al., Heart Rhythm.2022 Aug;19(8):1272-1280 [19].
5. Left bundle branch-optimized CRT
The results of BiVP have generally been disappointing when it is delivered to patients with intraventricular conduction delay (IVCD), which may be because BiVP does not reduce ventricular activation time in this group of patients. The effect of LBBP may also be limited in this group of patients, since many have preserved conduction via the left bundle, with prolongation in QRS occurring as a result of distal conduction system disease or myocardial disease. LBBP is unlikely to reduce ventricular activation time in these circumstances. Patients may also have mixed disease with block in the left bundle or one of its branches and more distal disease. In these patients LBBP may produce modest reductions in QRS duration.
Left bundle branch-optimized CRT (LOT-CRT) has been proposed as a pacing approach in these groups of patients. The concept of LOT-CRT was derived from HOT-CRT (His bundle pacing optimized CRT) [21]. LOT-CRT is delivered by pacing both the LBB and CS LV lead, with the aim of fusing left ventricular activation from the conduction system with the activation wavefront originating from the LV lead. The concept is that this combination may be more effective in reducing QRS duration than using either approach in isolation, in patients in where LBBP does not reduce or only produces modest reductions in QRS duration.
Recently, Jastrzebski M, et al. (2022) [22] reported the findings of an international multicenter observational study in which LOT-CRT was successfully delivered to 81% of a mixed group patients (LBBB 42%, IVCD 22%, RV pacing 23%, and right bundle branch block (RBBB) 12%). They observed that the LOT-CRT approach resulted in significantly greater narrowing of QRS complex from 182±25ms to 144±22ms compared to BiVP-CRT (170±30ms) and LBBAP (162±23ms). At follow-up of?>3 months, LVEF improved from 28.7±9.8% to 37±12%, NT-proBNP level decreased from 5821±8193pg/mL to 2514±3537pg/mL, and NYHA class improved from 2.9 to 1.9 in LOT-CRT. Therefore, the findings from this study suggest that LOT-CRT is a promising technique for delivering ventricular resynchronization, which may be particularly useful patients in whom LBBP does not reduce QRS duration i.e., IVCD or mixed disease with only modest QRS shortening with LBBP. Randomized controlled trials are justified to determine whether the LOT-CRT approach improves outcomes (Figure 5).
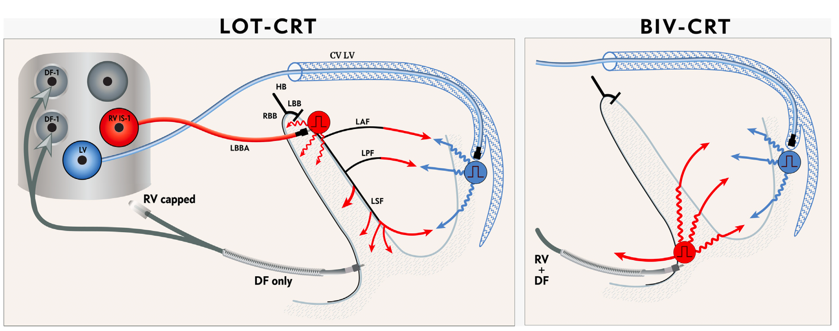
Figure 5: Schematic of pacing lead connection and left ventricular activation wavefronts of LOT-CRT compared with BiV-CRT.
CV LV=coronary venous left ventricular; DF=defibrillation; LAF=left anterior fascicle; LBB=left bundle branch; LBBA=left bundle branch area; LPF=left posterior fascicle; LSF=left septal fascicle; LV=left ventricle; RBB=right bundle branch; RV=right ventricle. Adapted from Jastrzebski M, et al., Heart Rhythm 2022 Jan;19(1):13-21 [20].
Further Questions Which Need to be Addressed
1. What is the optimal method for determining LBB capture and successful LBBB correction
The aim of LBBP is to capture the LBB, correct LBBB and restore LV synchrony. An important requirement when designing RCTs will be which criteria are used to confirm capture, since confirming conduction system capture will be important when assessing outcomes. There is currently no consensus on the optimal criteria for confirming left bundle capture.
Our approach is to use the following criteria [13], to confirm LBB capture in HF with LBBB: 1) R prime (RBBB pattern) in lead V1 during pacing, which implies that the lead is deep in the interventricular septum; 2) LVAT (measured from pacing stimulus to peak of R wave in V5 or V6) is ≤ 100ms at low pacing output (≤ 3 V/0.5ms); AND at least one of the following: a) abrupt shortening of LVAT by >10 ms during mid/deep septal lead placement with RBBB pattern in V1 at high output, and LVAT remains short and constant at high and low output with further advancement of the lead to final position; b) transition from nonselective to selective LBBP and no LVAT change at high and low outputs; c) transition from nonselective LBBP to left ventricular septal pacing (LVSP) (lengthening of LVAT by at least 10ms with or without obvious QRS morphology transition during threshold testing). Position is classified as LV septal pacing (LVSP) if LBBB capture cannot be confirmed using the above criteria.
Vijayaraman P, et al. (2021) [23] reported a novel criterion to diagnose LBB capture in patients with LBBB. They found an absolute value of 8ms for the difference in R wave peak time between HBP and LBBP in V6 (delta RWPT) provides 100% sensitivity and 93% specificity in confirming LBB capture. This method requires further validation, but has the potential to simplify the process of confirming left bundle capture. (Figure 6). Recently, our group also established a criterion of LVAT to determine LBB capture in HF patients with LBBB [24]. LVAT <85ms showed good predictive value for LBBP with a sensitivity of 84.0% and a specificity of 92.3%. The difference of LVAT during HBP and LBBP >9ms also exhibited 92.0% sensitivity and 92.3% specificity to predict successful LBBP. Huang Z, et al. (2021) reported that LVAT <85ms has 93% specificity and 76% sensitivity, and abrupt shortening of LVAT change >10ms has 100% specificity to confirm LBB capture [25].
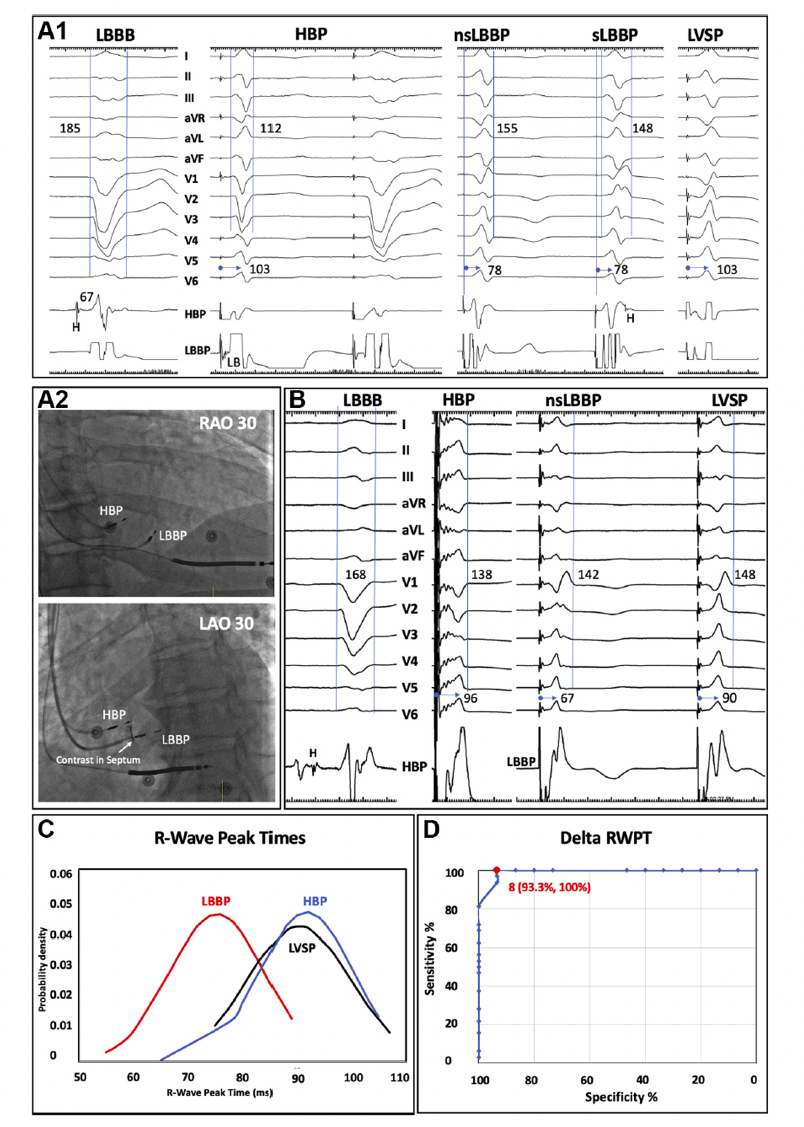
Figure 6: Novel criteria to diagnose LBB capture in patients with LBBB.
During corrective HBP, nonselective (ns) and selective (s) LBBP and LVSP, the R-wave peak times (RWPTs) are 103, 78, 78, and 103ms, respectively. The Delta RWPT of 25ms during LBBP and 0ms during LVSP correctly distinguishes LBB capture (A1). Right anterior oblique (RAO) and left anterior oblique (LAO) fluoroscopic views of HBP and LBBP leads of the patient are shown (A2). During corrective HBP, ns-LBBP, and LVSP, the RWPTs are 96, 67, and 90ms, respectively. The Delta RWPTs of 29ms during LBBP and 6ms during LVSP correctly distinguish LBB capture (B). Distribution curves of RWPTs during HBP, LBBP, and LVSP are shown (C). Receiver-operating characteristic curve for difference (delta) in RWPTs between HBP and LBBP/ LVSP is shown (D). LB=left bundle; LBBB=left bundle branch block. Adapted from Vijayaraman P. et al., JACC Clin Electrophysiol. 2021 Jun;7(6):808-810 [21].
2. Is Left ventricular septal pacing effective alternative to LBBP?
Mafi-Rad M, et al. (2016) [26] first described the feasibility and acute hemodynamic effects of LVSP using a transvenous approach through the interventricular septum. They showed LVSP can preserve acute LV pump function in patients with sinus node dysfunction and normal cardiac function. Salden F, et al. (2020) [27] reported that LVSP provided short-term hemodynamic improvement and similar electrical resynchronization compared to BiVP and HBP. This raises the question of whether conduction system capture is required or whether deep septal pacing could be sufficient to provide benefit. This would simplify the procedure. However, in a previous study [5] we observed that LBBP with clear evidence of direct LBB capture resulted in earlier activation of the left ventricular free wall compared to LVSP with only local myocardial capture. This finding suggest that left bundle capture is required to provide optimal ventricular resynchronization. Whether this superior resynchronization and more physiological ventricular activation pattern produces in differences in longer term outcomes requires further investigation.
3. Role of LBBP for patients with a bradycardia indication for pacing and impaired ventricular function
Previous studies have demonstrated that when RV myocardial pacing is delivered to patients with pre-existing LV impairment that it increases the risk of heart failure hospitalization and death [28]. The BLOCK-HF study [29] compared BiVP with RV pacing in patients with ventricular impairment (LVEF ≤ 50%) and AV block. They found that BiVP reduced the combined endpoint (time to death, urgent care visit for heart failure, or ≥15% increase in LV volume). The recent APAF-CRT study showed that BiVP-CRT combined with AV node ablation has been found to be beneficial in patients with persistent AF, and heart failure hospitalization in the preceding year [30]. However, as discussed above LBBP has physiological advantages compared to BiVP and we therefore believe that studies are justified to investigate whether LBBP improves outcomes in these groups of patients.
Conclusion
BiVP is an evidence-based therapy for selected patients with HF and conduction system disease. LBBP is a novel approach which can restore normal physiological left ventricular activation and thereby improve cardiac function. Observational studies have shown that LBBP is technically feasible in many patients, has low and stable capture thresholds and may improve clinical outcomes in patients with a CRT indication. A small pilot RCT found that might be more effective than BiVP in patients with non-ischemic cardiomyopathy and LBBB. These promising findings justify large RCTs to establish whether LBBP-CRT should become a first line method for delivering CRT.
References
1. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. New England Journal of Medicine. 2005 Apr 14;352(15):1539-49. https://doi.org/10.1056/NEJMoa050496
2. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M. Cardiac resynchronization in chronic heart failure. New England Journal of Medicine. 2002 Jun 13;346(24):1845-53. https://doi.org/10.1056/NEJMoa013168
3. Singh JP, Klein HU, Huang DT, Reek S, Kuniss M, Quesada A, Barsheshet A, Cannom D, Goldenberg I, McNitt S, Daubert JP. Left ventricular lead position and clinical outcome in the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation. 2011 Mar 22;123(11):1159-66. https://doi.org/10.1161/CIRCULATIONAHA.110.000646
4. Zhang J, Guo J, Hou X, Wang Y, Qian Z, Li K, Ge P, Zou J. Comparison of the effects of selective and non-selective His bundle pacing on cardiac electrical and mechanical synchrony. Ep Europace. 2018 Jun 1;20(6):1010-7. https://doi.org/10.1093/europace/eux120
5. Hou X, Qian Z, Wang Y, Qiu Y, Chen X, Jiang H, Jiang Z, Wu H, Zhao Z, Zhou W, Zou J. Feasibility and cardiac synchrony of permanent left bundle branch pacing through the interventricular septum. EP Europace. 2019 Nov 1;21(11):1694-702. https://doi.org/10.1093/europace/euz188
6. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, Mao G, Vijayaraman P, Ellenbogen KA. Long-term outcomes of His bundle pacing in patients with heart failure with left bundle branch block. Heart. 2019 Jan 1;105(2):137-43. https://doi.org/10.1136/heartjnl-2018-313415
7. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, Ellenbogen KA. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Canadian Journal of Cardiology. 2017 Dec 1;33(12):1736-e1. https://doi.org/10.1016/j.cjca.2017.09.013
8. Lustgarten DL, Crespo EM, Arkhipova-Jenkins I, Lobel R, Winget J, Koehler J, Liberman E, Sheldon T. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: a crossover design comparison. Heart rhythm. 2015 Jul 1;12(7):1548-57. https://doi.org/10.1016/j.hrthm.2015.03.048
9. Sharma PS, Dandamudi G, Herweg B, Wilson D, Singh R, Naperkowski A, Koneru JN, Ellenbogen KA, Vijayaraman P. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm. 2018 Mar 1;15(3):413-20. https://doi.org/10.1016/j.hrthm.2017.10.014
10. Huang W, Wu S, Vijayaraman P, Su L, Chen X, Cai B, Zou J, Lan R, Fu G, Mao G, Ellenbogen KA. Cardiac resynchronization therapy in patients with nonischemic cardiomyopathy using left bundle branch pacing. Clinical Electrophysiology. 2020 Jul 1;6(7):849-58. https://doi.org/10.1016/j.jacep.2020.04.011
11. Vijayaraman P, Ponnusamy S, Cano Ó, Sharma PS, Naperkowski A, Subsposh FA, Moskal P, Bednarek A, Dal Forno AR, Young W, Nanda S. Left bundle branch area pacing for cardiac resynchronization therapy: results from the International LBBAP Collaborative Study Group. Clinical Electrophysiology. 2021 Feb 1;7(2):135-47. https://doi.org/10.1016/j.jacep.2020.08.015
12. Chen X, Ye Y, Wang Z, Jin Q, Qiu Z, Wang J, Qin S, Bai J, Wang W, Liang Y, Chen H. Cardiac resynchronization therapy via left bundle branch pacing vs. optimized biventricular pacing with adaptive algorithm in heart failure with left bundle branch block: a prospective, multi-centre, observational study. EP Europace. 2022 May;24(5):807-16. https://doi.org/10.1093/europace/euab249
13. Wang Y, Zhu H, Hou X, Wang Z, Zou F, Qian Z, Wei Y, Wang X, Zhang L, Li X, Liu Z. Randomized trial of left bundle branch vs biventricular pacing for cardiac resynchronization therapy. Journal of the American College of Cardiology. 2022 Sep 27;80(13):1205-16. https://doi.org/10.1016/j.jacc.2022.07.019
14. Arnold AD, Shun-Shin MJ, Ali N, Keene D, Howard JP, Francis DP, Whinnett ZI. Contributions of Atrioventricular Delay Shortening and Ventricular Resynchronization to Hemodynamic Benefits of Biventricular Pacing. Clinical Electrophysiology. 2022 Sep 28. https://doi.org/10.1016/j.jacep.2022.07.024
15. Zhang W, Huang J, Qi Y, Wang F, Guo L, Shi X, Wu W, Zhou X, Li R. Cardiac resynchronization therapy by left bundle branch area pacing in patients with heart failure and left bundle branch block. Heart Rhythm. 2019 Dec 1;16(12):1783-90. https://doi.org/10.1016/j.hrthm.2019.09.006
16. Vijayaraman P, Zalavadia D, Haseeb A, Dye C, Madan N, Skeete JR, Vipparthy SC, Young W, Ravi V, Rajakumar C, Pokharel P. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm. 2022 Aug;19(8):1263-1271. https://doi.org/10.1016/j.hrthm.2022.04.023
17. Jiang Z, Wu T, Wu Y, Chen Z, Yang W, Chen C, Zhou X, Shan Q. Clinical Outcomes of Permanent Left Bundle Branch Area Pacing in Patients With Left Bundle Branch Block and Left Ventricular Ejection Fraction> 35 vs.≤ 35%. Frontiers in Cardiovascular Medicine. 2022;9. https://doi.org/10.3389/fcvm.2022.838708
18. Ye Y, Wu S, Su L, Sheng X, Zhang J, Wang B, Sharma PS, Ellenbogen KA, Su Y, Chen X, Fu G. Feasibility and outcomes of upgrading to left bundle branch pacing in patients with pacing-induced cardiomyopathy and infranodal atrioventricular block. Frontiers in cardiovascular medicine. 2021:550. https://doi.org/10.3389/fcvm.2021.674452
19. Qian Z, Wang Y, Hou X, Qiu Y, Wu H, Zhou W, Zou J. Efficacy of upgrading to left bundle branch pacing in patients with heart failure after right ventricular pacing. Pacing and Clinical Electrophysiology. 2021 Mar;44(3):472-80. https://doi.org/10.1111/pace.14147
20. Vijayaraman P, Herweg B, Verma A, Sharma PS, Batul SA, Ponnusamy SS, Schaller RD, Cano O, Molina-Lerma M, Curila K, Huybrechts W. Rescue left bundle branch area pacing in coronary venous lead failure or nonresponse to biventricular pacing: Results from International LBBAP Collaborative Study Group. Heart Rhythm. 2022 Aug;19(8):1272-1280. https://doi.org/10.1016/j.hrthm.2022.04.024
21. Vijayaraman P, Herweg B, Ellenbogen KA, Gajek J. His-optimized cardiac resynchronization therapy to maximize electrical resynchronization: a feasibility study. Circulation: Arrhythmia and Electrophysiology. 2019 Feb;12(2):e006934. https://doi.org/10.1161/CIRCEP.118.006934
22. Jastrz?bski M, Moskal P, Huybrechts W, Curila K, Sreekumar P, Rademakers LM, Ponnusamy SS, Herweg B, Sharma PS, Bednarek A, Rajzer M. Left bundle branch–optimized cardiac resynchronization therapy (LOT-CRT): results from an international LBBAP collaborative study group. Heart Rhythm. 2022 Jan 1;19(1):13-21. https://doi.org/10.1016/j.hrthm.2021.07.057
23. Vijayaraman P, Jastrzebski M. Novel criterion to diagnose left bundle branch capture in patients with left bundle branch block. Clinical Electrophysiology. 2021 Jun 1;7(6):808-10. https://doi.org/10.1016/j.jacep.2021.03.013
24. Qian Z, Xue S, Zou F, Qin C, Wang Y, Zhang X, Qiu Y, Wu H, Hou X, Zou J. New criterion to determine left bundle branch capture on the basis of individualized His bundle or right ventricular septal pacing. Heart Rhythm. 2022 Aug 3. https://doi.org/10.1016/j.hrthm.2022.07.022
25. Wu S, Chen X, Wang S, Xu L, Xiao F, Huang Z, Zheng R, Jiang L, Vijayaraman P, Sharma PS, Su L. Evaluation of the criteria to distinguish left bundle branch pacing from left ventricular septal pacing. Clinical Electrophysiology. 2021 Sep 1;7(9):1166-77. https://doi.org/10.1016/j.jacep.2021.02.018
26. Mafi-Rad M, Luermans JG, Blaauw Y, Janssen M, Crijns HJ, Prinzen FW, Vernooy K. Feasibility and acute hemodynamic effect of left ventricular septal pacing by transvenous approach through the interventricular septum. Circulation: Arrhythmia and Electrophysiology. 2016 Mar;9(3):e003344. https://doi.org/10.1161/CIRCEP.115.003344
27. Salden FC, Luermans JG, Westra SW, Weijs B, Engels EB, Heckman LI, Lamerichs LJ, Janssen MH, Clerx KJ, Cornelussen R, Ghosh S. Short-term hemodynamic and electrophysiological effects of cardiac resynchronization by left ventricular septal pacing. Journal of the American College of Cardiology. 2020 Feb 4;75(4):347-59. https://doi.org/10.1016/j.jacc.2019.11.040
28. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. Jama. 2002 Dec 1;288(24):3115-23. https://doi.org/10.1001/jama.288.24.3115
29. Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, Shinn T, St John Sutton M. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013 Apr 25;368:1585-93. https://doi.org/10.1056/NEJMoa1210356
30. Brignole M, Pentimalli F, Palmisano P, Landolina M, Quartieri F, Occhetta E, Calò L, Mascia G, Mont L, Vernooy K, Van Dijk V. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. European Heart Journal. 2021 Dec 7;42(46):4731-9. https://doi.org/10.1093/eurheartj/ehab569
