Atherosclerosis Assessment by Total-Body PET/CT
Authors: Poul F. Høilund-Carlsen1,2*, Reza Piri1,2, Oke Gerke1,2, Lars Edendbrandt3, Abass Alavi4
1Department of Nuclear Medicine, Odense University Hospital, Denmark
2Department of Clinical Research, University of Southern Denmark, Denmark
3Region Västra Götaland, Sahlgrenska University Hospital, Department of Clinical Physiology, Gothenburg, Sweden
4Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA
*Correspondence to: Poul F. Høilund-Carlsen, Department of Nuclear Medicine, Odense University Hospital, 5000 Odense C, Denmark; E-mail: pfhc@rsyd.dk
Received: October 10, 2021; Accepted: November 19, 2021; Published: November 28, 2021
Citation: Høilund-Carlsen PF, Piri R, Gerke O, et al., (2021) Atherosclerosis Assessment by Total-Body PET/CT. 21st Century Cardiol, Volume 1 (3): 112
Abstract
Atherosclerosis is mostly assessed by invasive angiography and/or noninvasive multislice CT, while MRI has been applied to characterize vulnerable coronary plaques, considered responsible for major cardiac events. These modalities demonstrate late-occurring atherosclerotic tissue changes, whereas PET/CT with 18F-fluorodeoxyglucose or 18F-sodium fluoride (NaF) depict early phase arterial inflammation and microcalcification, respectively, when the disease may be more responsive to therapy. The combination of the more promising tracer, NaF, total-body PET/CT imaging, and artificial intelligence-based scan interpretation appears to offer a more clinically useful assessment of arteriosclerotic disease burden for use in the diagnosis, treatment triage, and evaluation of therapy response.
Keywords:
Atherosclerosis; Cardiovascular disease; 18F-NaF; PET/CT; Total-body PET/CT; Artificial intelligence; Global disease score
Introduction
Much of what has been used to combat atherosclerosis over the past half-century are costly invasive procedures targeting acute or future major cardiovascular events. They are seldom applied before symptoms appear and, thus, cannot counteract or impede the underlying atherosclerosis process that has been going on for a decade or more. Management has gradually moved from invasive more towards medical therapy [1,2], typically in the shape of lifelong anti-cholesterol medication. Alongside, there has been a quest for methods that can identify and characterize so-called vulnerable or rupture-prone lesions in coronary, carotid, and other major arteries. However, none of the methods presented can predict with just a reasonable certainty which plaques will rupture and give rise to a cardiovascular event [3,4]. Moreover, there are indications that vulnerable plaques may not be as clinically important as previously thought, and that it might be fruitful to shift the focus from individual plaques to "atherosclerotic disease burden in coronary or overall cardiovascular risk assessment," as recently suggested by Arbab-Zadeh and Fuster [5].
Such a change opens for PET imaging, since with this modality it may be possible to detect the atherosclerotic process in its early phases, depict wherein the vascular system there is active, ongoing arteriosclerosis and measure the global arteriosclerotic burden in the heart and/or the major arteries and express this as a single Global Disease Score (GDS) [6,7]. A promising way to do this well and fast enough to be clinically useful is through Total-body PET/CT and artificial intelligence-based quantification of scans [8,9].
Atherosclerotic Disease Burden and Global Disease Score
Our understanding of atherosclerosis is constantly changing, especially influenced by experimental findings and more recently also in vivo imaging results that are constantly emerging, albeit with a focus on inflammation as the initiating factor ever since German pathologist Rudolf Virchow (1821-1901) first pointed to this possibility in the mid-19th century [10]. This perception has in recent decades created an intense interest in procedures able to detect and mitigate the risk associated with the vulnerable plaque, i.e., an accumulation of cholesterol and fatty debris under a thin fibrous sheath, which at some time may disintegrate and give rise to acute cardiovascular events [11,12]. Hence the alternative term ‘culprit lesion’, which by many is still considered the great villain in the arena, although, as mentioned, a state of generalized vulnerability may be more important than characterizing the individual sites of vulnerability in the individual patient, since reports have demonstrated that plaque rupture often occurs without clinical symptoms, plaque morphology changes over a few months, and plaque rupture frequently occurs apart from the culprit lesions [5].
PET Imaging of Atherosclerosis
PET imaging of atherosclerosis was initiated 20 years ago by Dr. Abass Alavi and his group at the University of Pennsylvania. They used 18F-fluorodeoxyglucose (FDG) to image arterial wall inflammation [13]. 18F-sodium fluoride (NaF) imaging was introduced about 10 years later by Derlin and co-workers in Germany to demonstrate arterial wall microcalcification [14]. Since then, differences between the two tracers in imaging of atherosclerosis have emerged [3,4]: (1) arterial uptakes of FDG and NaF seldom overlap in site and time; (2) FDG uptake rarely co-localizes with CT-detectable calcification, whereas NaF uptake often does; (3) FDG uptake does not correlate with common risk factors, while NaF uptake does; (4) FDG uptake in coronary arteries cannot be properly measured due to physiologic myocardial uptake. Due to these and other differences in favor of NaF, the interest has shifted towards further use of this tracer. The knowledge gained so far can be summarized as follows:
- Arterial wall inflammation and microcalcification are dynamic processes that are not directly interconnected.
- Atherosclerosis is a more dynamic and more influential process than hitherto thought.
- NaF-avid microcalcification can occur in fatty streaks, but the degree of progression to CT-calcification is unknown.
- Arterial NaF uptake often presents before CT-calcification, tends to decrease with increasing density of CT-calcification, and appears, rather than FDG-avid foci, to progress to CT-detectable calcification.
- NaF-PET/CT offers an individualized measurement of microcalcification in the heart, aorta, carotids, and other major arteries with reproducibility allowing for monitoring of anti-atherosclerotic intervention.
- Total body PET provides unique opportunities for studying atherosclerosis and its management more profoundly due to much higher sensitivity, ultra-short acquisition, and minimal radiation to the patient; this allows for disease screening, delayed and repeat imaging with features such as global disease scoring and parametric imaging to characterize the individual patient much better than hitherto seen.
Uncertainties
- Arterial 18F-fluorodeoxyglucose uptake appears to come and go with time (months); preliminary data suggest that 18F-sodium fluoride uptake may also be a more varying process than previously anticipated; longitudinal studies are warranted for clarification.
- Little is known about the potential transition in humans of active arterial wall calcification assessed by NaF-PET/CT to less active or consolidated calcification detected by CT: Is NaF uptake always or only sometimes a precursor to these?
- The question of whether early-phase atherosclerosis and calcification can be modified is unanswered due to the lack of intervention studies.
The goal is to provide a single number, the GDS, for the arteriosclerosis burden and use this to characterize the individual patient accurately and to assess whether prevention and treatment have the desired effect (9).
Total-Body PET/CT and Total-Body Atherosclerosis Assessment
With the advent of long-axial PET scanners that allow an elongated axial field of view of 70 or up to 200 cm, exemplified by the PennPET Explorer [15,16] and the United Imaging Explorer scanner (Figure 1) [17,18] respectively, it has become possible to collect dynamic and quantitative information on illness in major parts or all over the body in a way and with a speed that has never been possible before.
Figure 1: The uEXPLORER Total-Body PET/CT scanner produced by United Imaging Healthcare, Shanghai, in partnership with the EXPLORER Consortium led by Simon Cherry and Ramsey Badawi from the University of California, Davis. uEXPLORER is the world's first medical imaging scanner that can capture a 3D picture of the whole hu-man body at one bed position (Courtesy: United Imaging Healthcare America, Inc.).
The PET part of these instruments has much higher sensitivity than current PET/CT scanners allowing a large part of or total body acquisitions in a few minutes with the same, fixed bed position. This allows delayed imaging when this is preferable, improved motion correction, full-body recordings to elucidate disease activity in more than one or just a few locations in the body, and dynamic recordings enabling parametric imaging for kinetic analysis to provide valuable estimates of metabolism, oxygen use, signal transduction, and pharmacodynamics that have previously not been available in the routine clinical setting [9,15-18]. Successive scans of the same patient are no longer a problem from the point of view of radiation hygiene and will allow for multiple diseases and therapy monitoring scans. Summing up, an entirely new series of possibilities open up with total-body PET imaging [9,19,20], e.g.:
- Mapping and quantification of atherosclerosis, its location, and relative activity throughout the body, something that may have both prognostic and therapeutic implications.
- Screening for incipient, but threatening atherosclerotic processes in asymptomatic patients or patients with uncharacteristic symptoms including cerebral atherosclerosis, carotid atherosclerosis as a forerunner of stroke, and accelerated cardiac, aortic, or peripheral arterial disease, all of which may be sensitive to early-onset therapy.
- Disease characterization with several different PET tracers in the same patient.
- Easy, fast, and risk-free monitoring of a variety of therapeutic interventions even in the same individual.
- Calculation of a single score, the GDS, expresses the atherosclerotic burden in the body and its activity at diagnosis and as an easy, simple, and reliable guidance for therapy.
Artificial Intelligence in Vascular PET Imaging
Calculation of the GDS for any disease is very cumbersome, time-consuming, and sometimes quite impossible. This is where AI-based analysis of PET/CT scans is going to play a significant role in a wide range of diseases. For quantitative analysis, we have used the Swedish “Research Consortium for Medical Image Analysis (RECOMIA)” platform with great success [21]. Assessment of atherosclerosis cannot be done properly by visual inspection and manual grading of arterial wall tracer uptake. These procedures are much too uncertain in that early changes are of-ten diffuse and non-visible and because manual segmentation of the vascular system is more observer-dependent and very time-consuming. Analysis using manual segmentation for quantifying a single PET/CT scan for atherosclerosis may easily last 1-2 hours compared to less than a minute with the AI-based technique. Recently, it has been introduced for assessment of atherosclerosis, showing very promising results despite a small number of training scans, indicating that it will very quickly develop further as the number of training examples from clinically diverse patients is constantly increasing [22,23]. Atherosclerosis in other parts of the body than the heart and large arteries (Figure 2), like the brain and lungs [24,25] can now be included in the overall clinical picture. AI-based algorithms allow for consideration also of a wide range of other clinical and paraclinical parameters in a way that the individual physician cannot possibly do, all of which infer that the AI-based approach has come to stay and will soon be indispensable [9].
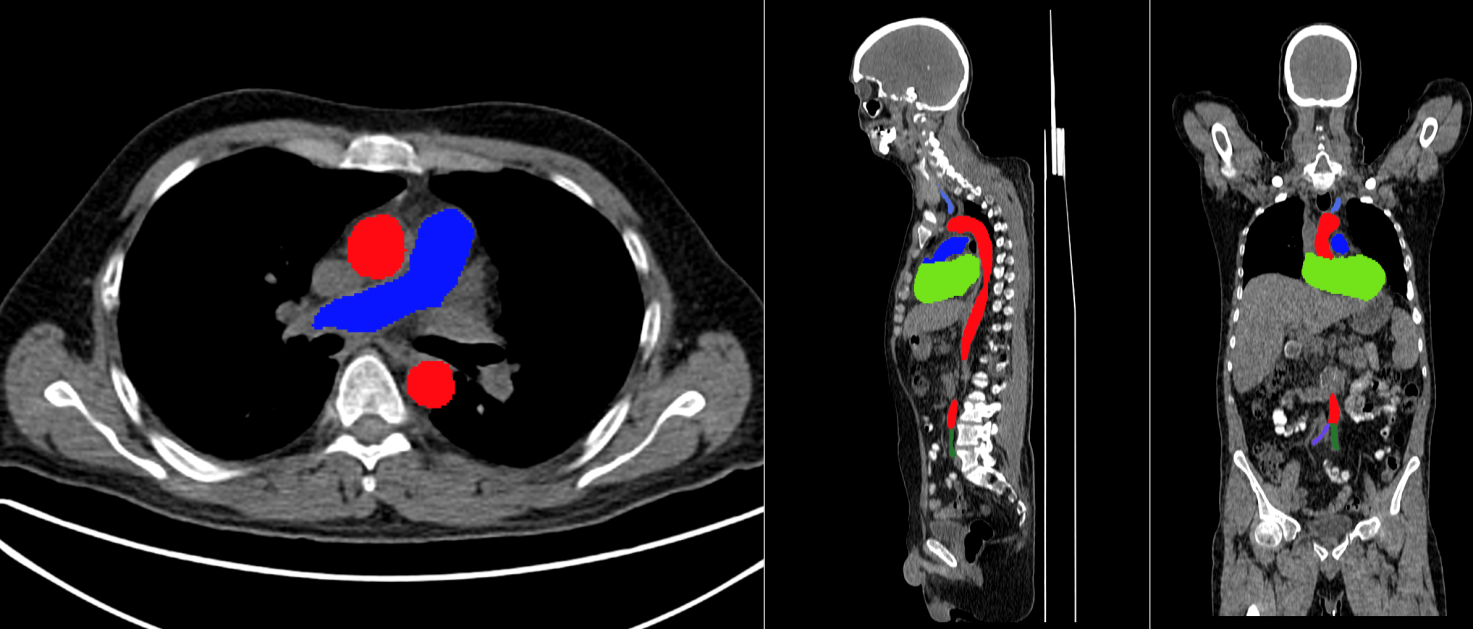
Figure 2: Axial, sagittal and coronal (from left to right) reconstruction of AI-based segmentation of the heart, aorta, and major arteries performed on the RECOMIA platform: https://www.recomia.org/. Color coding: Aorta – red, pulmonary artery – blue, right carotid – green (hardly visible in chosen coronal slice), left carotid – blue, right common iliac – blue, left common iliac – green.
Conclusion
Total-body PET/CT imaging of arteriosclerosis is a promising new opportunity to detect and measure atherosclerosis burden in its early active stages in such a fast, accurate, and reproducible way that this technique may not only change our perception of the disease atherosclerosis but also radically improve its management.
Acknowledgement
There was no funding provided for this paper.
Conflicts of Interest
The authors of this article have no conflicts to disclose.
References
1. Libby P, Buring JE, Badimon L, et al. (2019) Atherosclerosis. Nat Rev Dis Primers 5: 56. https://doi.org/10.1038/s41572-019-0106-z
2. Libby P (2021) The changing landscape of atherosclerosis. Nature 592: 524-533. https://doi.org/10.1038/s41586-021-03392-8
3. Høilund-Carlsen PF, Sturek M, Alavi A, et al. (2020) Atherosclerosis imaging with 18F-sodium fluoride PET: state-of-the-art review. Eur J Nucl Med Mol Imaging 47: 1538-1551. https://doi.org/10.1007/s00259-019-04603-1
4. Høilund-Carlsen PF, Piri R, Constantinescu C, et al. (2020) Atherosclerosis Imaging with 18F-Sodium Fluoride PET. Diagnostics (Basel) 10: 852. https://doi.org/10.3390/diagnostics10100852
5. Arbab-Zadeh A, Fuster V (2015) The myth of the "vulnerable plaque": transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol 65: 846-855. https://doi.org/10.1016/j.jacc.2014.11.041
6. Høilund-Carlsen PF, Edenbrandt L, Alavi A (2019) Global disease score (GDS) is the name of the game! Eur J Nucl Med Mol Imaging 46: 1768-1772. https://doi.org/10.1007/s00259-019-04383-8
7. Saboury B, Edenbrandt L, Piri R, et al. (2021) Alavi-Carlsen Calcification Score (ACCS): a sim-ple measure of global cardiac atherosclerosis burden. Diagnostics (Basel) 11: 1421. https://doi.org/10.3390/diagnostics11081421
8. Borja AJ, Rojulpote C, Hancin EC, et al. (2020) An update on the role of total-body pet imaging in the evaluation of atherosclerosis. PET Clin 15: 477-485.
https://doi.org/10.1016/j.cpet.2020.06.006
9. Høilund-Carlsen PF, Piri R, Gerke O, et al. (2021) Assessment of total-body atherosclerosis by pet/computed tomography. PET Clin 16: 119-128. https://doi.org/10.1016/j.cpet.2020.09.013
10. Minelli S, Minelli P, Montinari R (2020) Reflections on atherosclerosis: Lesson from the past and future. J Multidiscip Healthc 13: 621-633. https://doi.org/10.2147/JMDH.S254016
11. Fuster V (1994) Lewis A. Conner memorial lecture. mechanisms leading to myocardial in-farction: insights from studies of vascular biology. Circulation 90: 2126-2146. https://doi.org/10.1161/01.cir.90.4.2126
12. Schroeder AP, Falk E (1995) Vulnerable and dangerous coronary plaques. Atherosclerosis 118: S141-S149. https://doi.org/10.1016/0021-9150(95)90081-0
13. Yun M, Yeh D, Araujo LI, et al. (2001) F-18 FDG uptake in the large arteries: a new observation. Clin Nucl Med 26: 314-319. https://doi.org/10.1097/00003072-200104000-00007
14. Derlin T, Richter U, Bannas P, et al. (2010) Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med 51: 862-865. https://doi.org/10.2967/jnumed.110.076471
15. Cherry SR, Badawi RD, Karp JS, et al. (2017) Total-body imaging: Transforming the role of positron emission tomography. Sci Transl Med 9: eaaf6169. https://doi.org/10.1126/scitranslmed.aaf6169
16. Pantel AR, Viswanath V, Daube-Witherspoon ME, et al. (2020) PennPET explorer: Human imaging on a whole-body imager. J Nucl Med 61: 144-151. https://doi.org/10.2967/jnumed.119.231845
17. Badawi RD, Shi H, Hu P, et al. (2019) first human imaging studies with the EXPLORER total-body pet scanner. J Nucl Med 60: 299-303. https://doi.org/10.2967/jnumed.119.226498
18. Zhang X, Xie Z, Berg E, et al. (2020) Total-body dynamic reconstruction and parametric imaging on the uEXPLORER. J Nucl Med 61: 285-291. https://doi.org/10.2967/jnumed.119.230565
19. Saboury B, Morris MA, Farhadi F, et al. (2020) Reinventing molecular imaging with total-body PET, Part I: technical revolution in evolution. PET Clin 15: 427-438. https://doi.org/10.1016/j.cpet.2020.06.012
20. Saboury B, Morris MA, Nikpanah M, et al. (2020) Reinventing molecular imaging with total-body PET, Part II: clinical applications. PET Clin 15: 463-475. https://doi.org/10.1016/j.cpet.2020.06.013
21. Trägårdh E, Borrelli P, Kaboteh R, et al. (2020) RECOMIA-a cloud-based platform for artificial intelligence research in nuclear medicine and radiology. EJNMMI Phys 7:51. https://doi.org/10.1186/s40658-020-00316-9
22. Piri R, Edenbrandt L, Larson M, et al. (2021) "Global" cardiac atherosclerotic burden assessed by artificial intelligence based versus manual segmentation in 18F-sodium fluoride PET/CT scans: head to head comparison. J Nucl Cardiol (Inpress) https://doi.org/10.1007/s12350-021-02758-9
23. Piri R, Edenbrandt L, Larsson M, et al. (2021) Aortic wall segmentation in 18F-sodium fluoride PET/CT scans: head-to-head comparison of artificial intelligence-based versus manual segmentation. J Nucl Cardiol (Inpress). https://doi.org/10.1007/s12350-021-02649-z
24. Al-Zaghal A, Mehdizadeh Seraj S, Werner TJ, et al. (2018) Assessment of physiological intracranial calcification in healthy adults using 18F-NaF PET/CT. J Nucl Med 60: 267-271. https://doi.org/10.2967/jnumed.118.213678
25. Al-Zaghal A, Aras M, Borja AJ, et al. (2020) Detection of pulmonary artery atherosclerosis by FDG-PET/CT: a new observation. Am J Nucl Med Mol Imaging 10: 127-134.
26. Leung EK, Berg E, Omidvari N, et al. (2021) Quantitative accuracy in total-body imaging using the uEXPLORER PET/CT scanner. Phys Med Biol 66: 205008. https://doi.org/10.1088/1361-6560/ac287c
27. Saboury B, Edenbrandt L, Piri R, et al. (2021) Alavi-Carlsen Calcification Score (ACCS): a simple measure of global cardiac atherosclerosis burden. Diagnostics (Basel) 11: 1421. https://doi.org/10.3390/diagnostics11081421
28. Hu Y, Liu G, Yu H, et al. (2021) Feasibility of ultra-low (18)F-FDG activity acquisitions using total body PET/CT. J Nucl Med 62: jnumed.121.262038. https://doi.org/10.2967/jnumed.121.262038
29. Moskal P, St?pie? E? (2020) Prospects and clinical perspectives of total-body PET imaging using plastic sintillators. PET Clin 15: 439-452. https://doi.org/10.1016/j.cpet.2020.06.009
