Are New Cancer Therapies the Death of Haematopoietic Cell Transplants for Acute Myeloid Leukaemia?
Running Title: Transplant Obituary?
Authors: Robert Peter Gale1*, Gordon Phillips2, and Hillard M Lazarus3
1Centre for Haematology Research, Department of Immunology and Inflammation, Imperial College London, London, UK
2Wake Forest Comprehensive Cancer Center, Winston-Salem, NC, USA
3Department of Medicine, Case Western Reserve University, Cleveland, OH, USA
*Correspondence to: Professor. Robert Peter Gale, MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Centre for Haematology Research, Department of Immunology and Inflammation, Imperial College London, London, UK; E-mail: robertpetergale@alumni.ucla.edu
>Received: July 15, 2021; Revision: August 10, 2021; Accepted: August 13, 2021; Published: August 20, 2021
Citation: Gale RP, Phillips G, Lazarus HM (2021) Are New Cancer Therapies the Death of Haematopoietic Cell Transplants for Acute Myeloid Leukaemia? 21st Century Pathol, Volume 1 (1): 101
Abstract
There are many new drug approvals for cancers for which haematopoietic cell transplants are sometimes considered a standard-of-care under certain circumstances including auto transplants for Plasma Cell Myeloma (PCM) and Non-Hodgkin Lymphomas (NHL) and allotransplants for Acute Myeloid Leukaemia (AML), Acute Lymphoblastic Leukaemia (ALL) and Myelodysplastic Syndromes (MDS). We focus on new drug approvals for AML and discuss whether these drugs will reduce use of allotransplants. Another important consideration is number of persons with AML able to receive an allotransplant is increasing because of the development of reduced-intensity pretransplant conditioning regimens and use of HLA-haplotype-matched relatives as donors with post-transplant cyclophosphamide. Also, better donor selection, immune suppressive drugs, antibiotics and supportive care have resulted in a 20% reduction in early deaths and a 10% long-term survival increase. We conclude that although these new drugs may, inappropriately, make more people with AML transplant-eligible, the net impact of these advances on transplant rates is likely to be small.
Keywords:
Haematopoietic cell transplants; Immune therapy; Targeted therapy; Acute leukaemia
Short Communication
The first successful haematopoietic cell transplants were in 1956. Increased understanding of transplant biology, histocompatibility testing, immune-suppressive drugs and better supportive care made transplants more widely available with over 45,000 transplants done annually globally. There are now more than 1.5 million transplant recipients worldwide [1]. In the US in 2018 approximately 14,000 auto transplants and 9,000 allotransplants were done with annual rates increasing by about 3 percent [2]. In the US most autotransplants are for plasma cell myeloma (PCM) and non-Hodgkin lymphomas (NHL) whereas most allotransplants are done for acute myeloid leukaemia (AML) and myelodysplastic syndromes (MDS). Relatively few allotransplants are for acute lymphoblastic leukaemia (ALL).
There have been several recent advances in therapy of haematologic cancers including new drugs and targeted and immune therapies which raise the question of whether transplants will remain an important therapy intervention [3].
We display show the pace of recent FDA drug approvals in AML in Figure 1. This accelerated progress is encouraging but it is important to consider 3 caveats: (1) Most persons benefiting from these newly-approved drugs would not have been transplant candidates; (2) The effect size of new drugs is modest and rarely curative; and (3) In SEER data 5-year survival of persons with AML has only improved 10 percent in the most recent data [4].
Also, in the past decade, several so-called targeted therapies for myeloid cancers have been developed including midostaurin, sorafenib, gilteritinib, enasidenib, ivosidenib, ruxolitinib, and fedratinib. Again, this is welcome progress but the same 3 caveats apply to targeted therapies. We estimate only about 10 percent of persons will benefit from targeted therapies [5].
Immune therapy is another new strategy to treat haematologic cancers. Examples include monoclonal antibodies (such as rituximab, daratumumab), antibody-drug conjugates (such as brentuximab), bi- or tri-specific antibodies (such as blinatumomab), Chimeric Antigen Receptor (CAR)-T-cells (such as axicabtagene ciloleucel, tisagenlecleucel, idecabtagene vicleucel, and brexucabtagene autoleucel) and immune checkpoint inhibitors (such as nivolumab, pembrolizumab, and ipilimumab) [6]. These drugs are effective predominately in B-cell cancers and are lineage-, not cancer-specific. Some are strikingly effective in producing remission but less than one-half of these are long-term and many people including complete responders eventually receive a transplant. There is no proved safe and effective immune therapy of AML other than gemtuzumab ozogamicin which is rarely used [7].
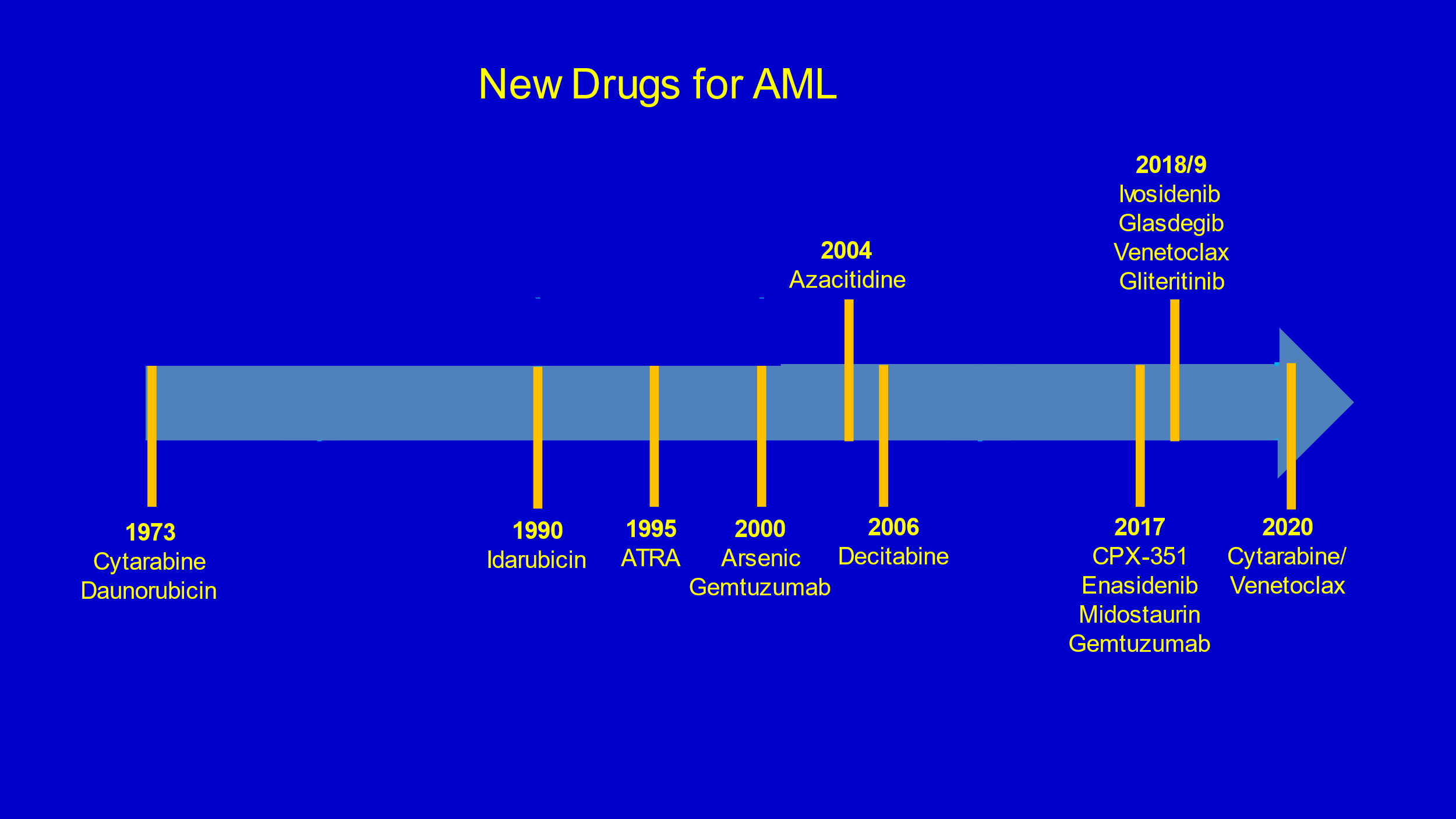
Figure 1: Recent FDA drug approval in AML.
Because these new therapies achieve remissions in persons with advanced cancer they are likely to increase numbers of persons considered transplant-eligible. This will likely be so but we caution the notion transplants should only be reserved for people in remission is unsupported by convincing data. Although outcomes of transplant are better in persons in remission compared with those not in remissions, the relative benefit compared with alternative therapy is not different and the better outcomes persons in remission predominately derives from subject selection biases.
It is important to recall that much of the efficacy of allotransplants results from an allogeneic anti-cancer effect so far difficult to distinguish from graft-versus-host disease (GvHD) [8,9]. This allogeneic anti-cancer effect does not operate in the context of current cell-based immune therapies such as autologous CAR-T- or NK-cells.
Another important consideration is number of persons able to receive an allotransplant is increasing because of the development of reduced-intensity pretransplant conditioning regimens and the use of HLA-haplotype-matched relative?s as donors with post-transplant cyclophosphamide.
Also, better donor selection, immune-suppressive drugs, antibiotics, and supportive care have resulted in a 20 percent reduction in early deaths and a 10% long-term survival increase [10].
The advances we cite above in hematologic cancers are important and exciting but the magnitude of benefit is modest and few people are cured. Some of these therapies are in their infancy and long-term safety and efficacy are unknown. For the reasons we discuss we believe transplants will remain an important therapy soon. We are still not curing most people with these cancers and the challenge is how to best combine these new therapies with transplants. The bottom line is don?t disband your transplant team yet and/or fire your transplant specialists. After all, you may need them to give CAR-T-cells and the like.
Conclusion
We discuss results of new therapies of haematologic cancers. We show most improve outcomes only modestly, are not usually curative, are used predominately in persons not transplant candidates and report only short-term outcomes and, as such, are unlikely to replace transplants in the near future. We suggest transplants are likely to be used together with these new therapies to treat haematologic cancers. We believe transplants will remain an important therapy in the near future. The bottom line is don?t disband your transplant team yet and/or fire your transplant specialists. You may need them to give CAR-T-cells and the like.
Acknowledgement
RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Conflict of Interest
RPG is a consultant to BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Pharmaceuticals Inc. CStone Pharmaceuticals, NexImmune Inc. and Prolacta Bioscience; advisor to Antegene Biotech LLC, Medical Director, FFF Enterprises Inc.; partner, AZAC Inc.; Board of Directors, Russian Foundation for Cancer Research Support; and Scientific Advisory Board: StemRad Ltd. GLP is or has been a consultant or advisor to Novartis, Amgen, Ariad/Takeda, Astellas, Celgene/BMS, CVS/Caremark, Epizyme and MorphoSys, and has received clinical research support from Novartis, Astellas, Celgene, Cellectis, Daiichi Sankyo, Forty Seven, Rafael Pharmaceuticals, and royalties from UpToDate. HML has been a consultant for Partner Therapeutics, Jazz Pharmaceuticals, Seattle Genetics, AstraZeneca, Celgene/Bristol-Myers Squibb and Actinium Pharmaceuticals.
References
1. Niederwieser D, Baldomero H, Atsuta Y, et al. (2019) One and half million hematopoietic stem cell transplants (HSCT). Dissemination, trends and potential to improve activity by telemedicine from Worldwide Network for Blood and Marrow Transplantation (WBMT). Blood 134:2035. https://doi.org/10.1182/blood-2019-125232
2. COVID-19 Updates from CIBMTR. https://www.cibmtr.org/Pages/index.aspx
3. Gale RP, Phillips GL 2nd, Lazarus HM (2021) New cancer therapies. Are haematopoietic cell transplants a dead duck? Bone Marrow Transplant 56: 1086-1089. https://doi.org/10.1038/s41409-020-01151-3
4. Acute Myeloid Leukemia-Cancer Stat Facts (2021) Cancer Stat Facts: Leukemia-Acute Myeloid Leukemia (AML). National Cancer Institute. https://seer.cancer.gov/statfacts/html/amyl.html
5. Prasad V, Gale RP (2016) Precision medicine in acute myeloid leukemia: Hope, hype or both? Leuk Res 48: 73-77. https://doi.org/10.1016/j.leukres.2016.07.011
6. Boyiadzis M, Bishop MR, Abonour R, et al. (2016) The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of hematologic malignancies: multiple myeloma, lymphoma, and acute leukemia. J Immunother Cancer 4: 90.
https://doi.org/10.1186/s40425-016-0188-z
7. Godwin CD, Gale RP, Walter RB (2017) Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia 31: 1855-1868. https://doi.org/10.1038/leu.2017.187
8. Horowitz MM, Gale RP, Sondel PM, et al. (1990) Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75: 555-562. PMID: 2297567
9. Gale RP, Fuchs EJ (2016) Is there really a specific graft-versus-leukaemia effect? Bone Marrow Transplant 51: 1413-1415. https://doi.org/10.1038/bmt.2016.183
10. McDonald GB, Sandmaier BM, Mielcarek M, et al. (2020) Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003-2007 versus 2013-2017 cohorts. Ann Intern Med 172: 229-239. https://doi.org/10.7326/m19-2936
